【International Papers】Chemical reaction mechanism between trimethylgallium and oxygen for β-gallium oxide growth: Thermodynamic and experimental studies
日期:2025-09-19阅读:360
Researchers from the Lund University have published a dissertation titled "Chemical reaction mechanism between trimethylgallium and oxygen for β-gallium oxide growth: Thermodynamic and experimental studies" in Journal of Applied Physics.
Background
Thermodynamic analysis is widely recognized as a powerful tool for understanding chemical reactions and identifying optimal conditions on crystal growth systems, both in conventional III–V compound semiconductor and in oxide semiconductor. In particular, the growth behavior of β-Ga2O3 using HVPE and MBE methods, as well as using MOCVD within the TEG-O2 system, has been well-studied and clarified. However, unlike for the TEGa–O2 system, the chemical reaction dynamics between TMGa and O2 have not been studied in detail, and there are no clear guidelines for identifying optimal growth conditions. In this study, we performed a thermodynamic analysis of β-Ga2O3 MOCVD using TMGa and O2 as precursors, to clarify the chemical reaction dynamics and identify the preferred growth conditions. The experimental results confirmed that the observed behavior aligns with thermodynamic predictions.
Abstract
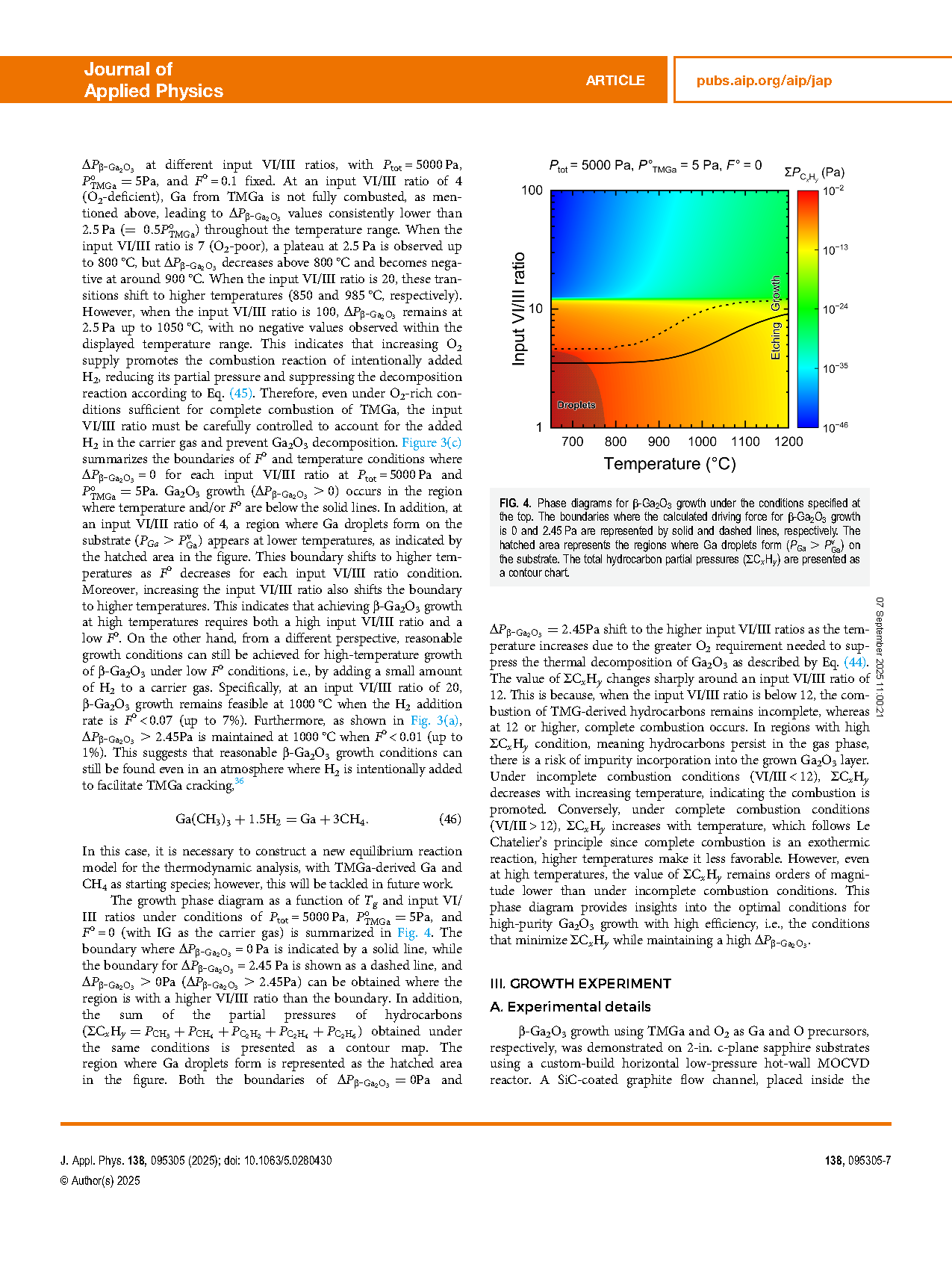
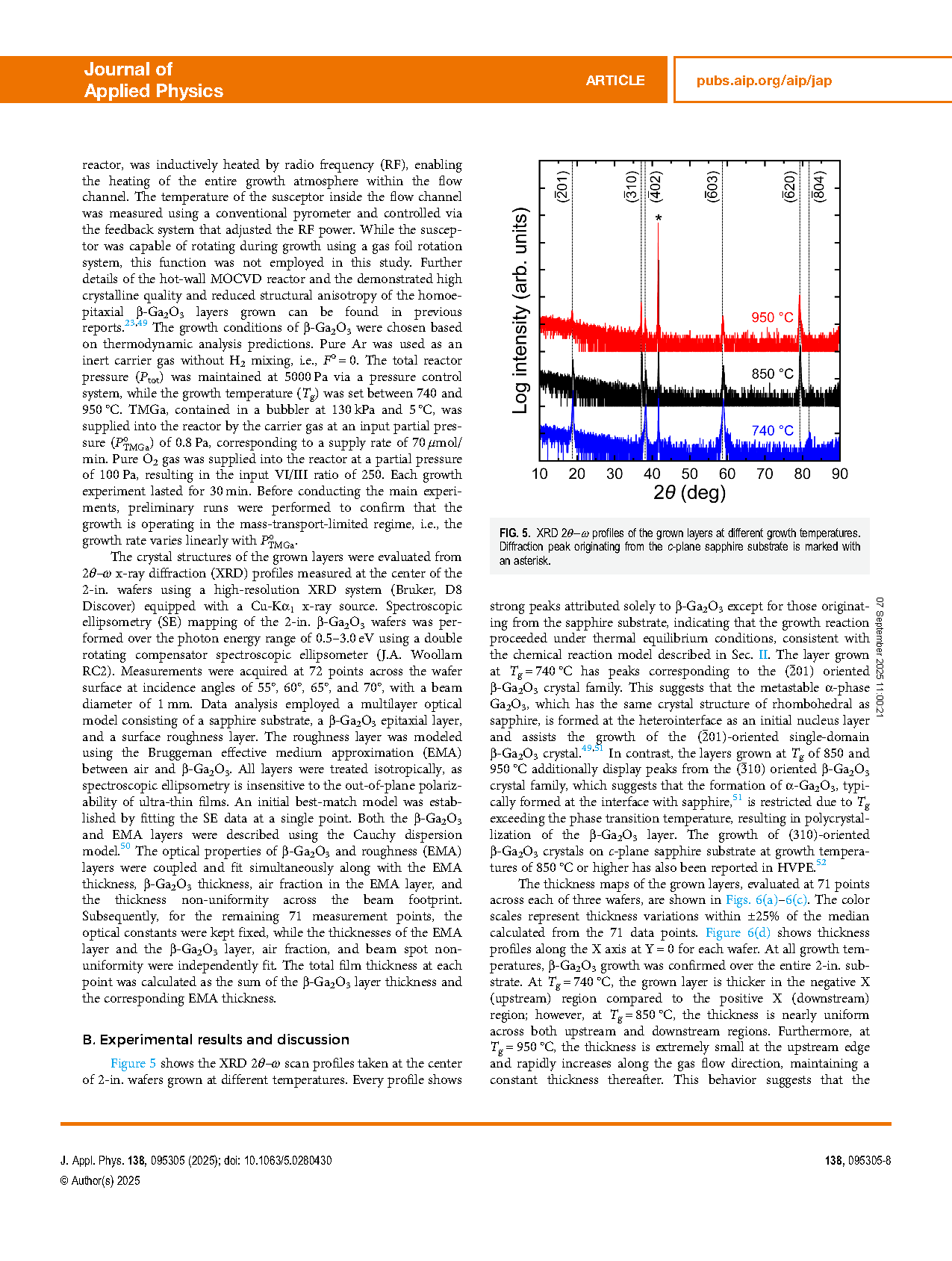
The metalorganic chemical vapor deposition (MOCVD) dynamics of beta-gallium oxide (β-Ga2O3) growth using trimethylgallium (TMGa) and oxygen as precursors were investigated through both theoretical thermodynamic analysis and experimental validation in a horizontal low-pressure hot-wall reactor. Thermodynamic analysis revealed that high-purity β-Ga2O3 can be grown through the complete combustion of TMG-derived gallium and hydrocarbons. Furthermore, the complete combustion of intentionally supplied hydrogen into the growth system also prevents the degradation of β-Ga2O3 growth. Therefore, a high input VI/III ratio that ensures full combustion of gaseous species in the growth system is preferred for β-Ga2O3 MOCVD. The growth experiments were performed on 2-in. sapphire substrates under an input VI/III ratio of 250. β-Ga2O3 growth was confirmed at growth temperatures between 740 and 950 °C, with the growth rate decreasing from 0.7 to 0.5 μm/h as temperature increased. Thermodynamic analysis successfully reproduced this growth behavior under the effective VI/III ratio of 6.5. The results demonstrate that β-Ga2O3 MOCVD occurred under thermal equilibrium conditions and was thermodynamically controllable.
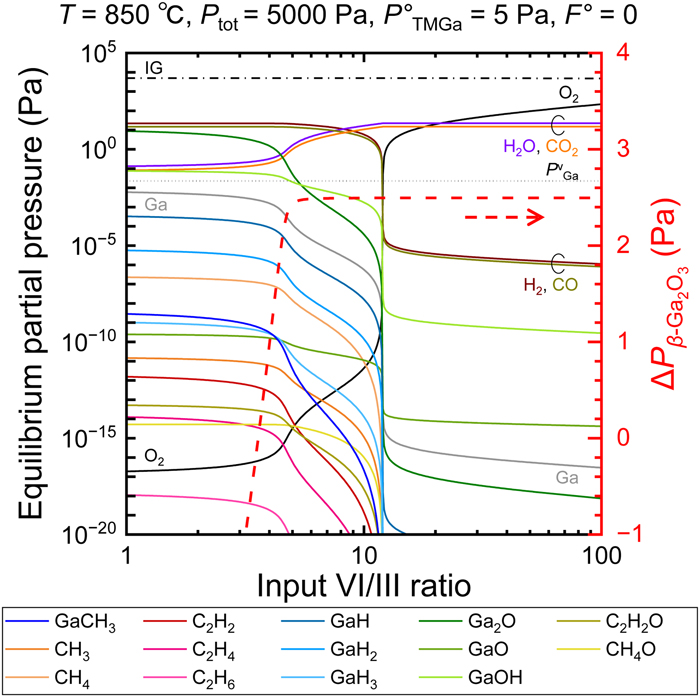
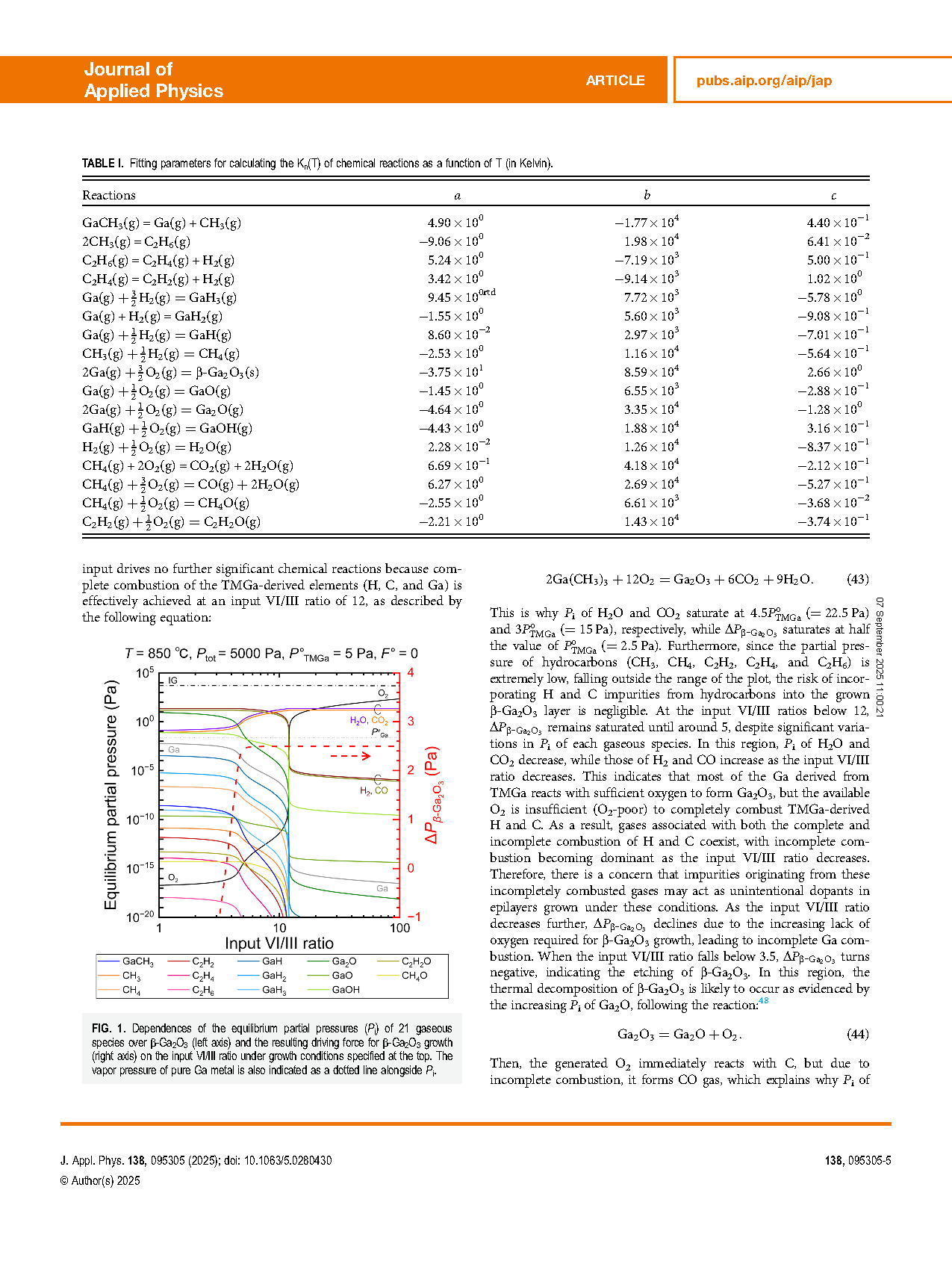
FIG.1 Dependences of the equilibrium partial pressures (Pi) of 21 gaseous species over β-Ga2O3 (left axis) and the resulting driving force for β-Ga2O3 growth (right axis) on the input VI/III ratio under growth conditions specified at the top. The vapor pressure of pure Ga metal is also indicated as a dotted line alongside Pi
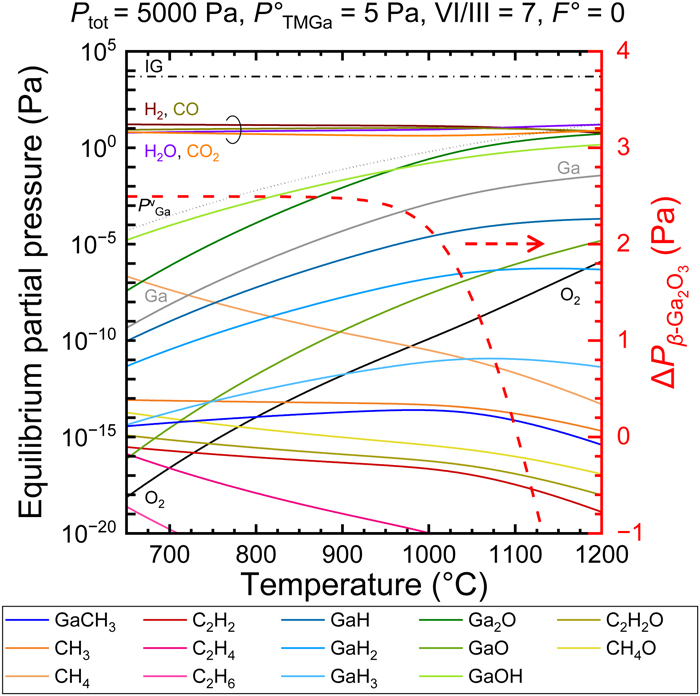
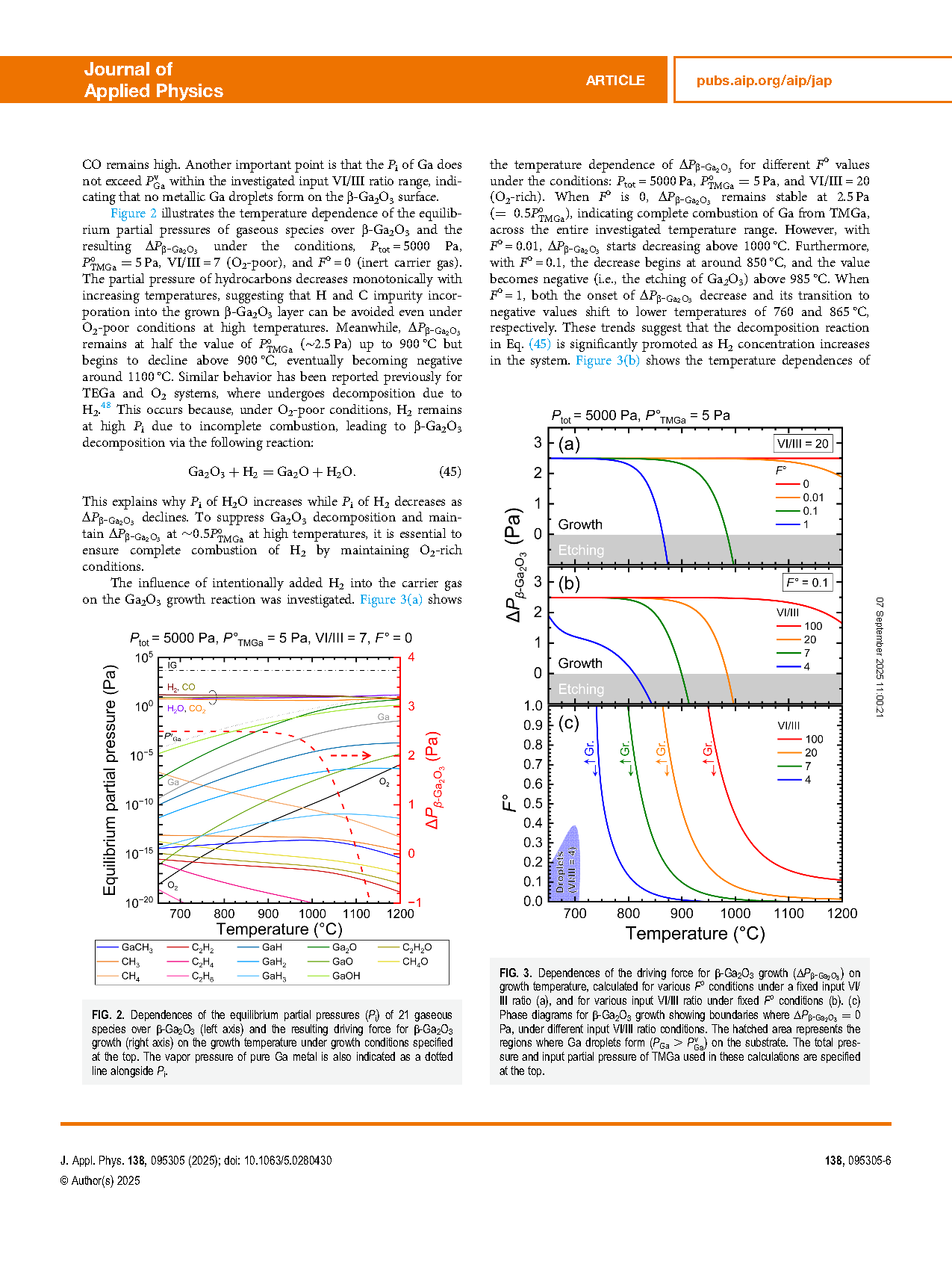
FIG.2 Dependences of the equilibrium partial pressures (Pi) of 21 gaseous species overβ-Ga2O3 (left axis) and the resulting driving force forβ-Ga2O3 growth (right axis) on the growth temperature under growth conditions specified at the top. The vapor pressure of pure Ga metal is also indicated as a dotted line alongside Pi.
DOI:
doi.org/10.1063/5.0280430