【International Papers】High-Entropy Liquid Metal Process for Transparent Ultrathin p-Type Gallium Oxide
日期:2025-04-03阅读:566
Researchers from the University of New South Wales (UNSW) have published a dissertation titled "High-Entropy Liquid Metal Process for Transparent Ultrathin p-Type Gallium Oxide" in Advanced Functional Materials.
Abstract
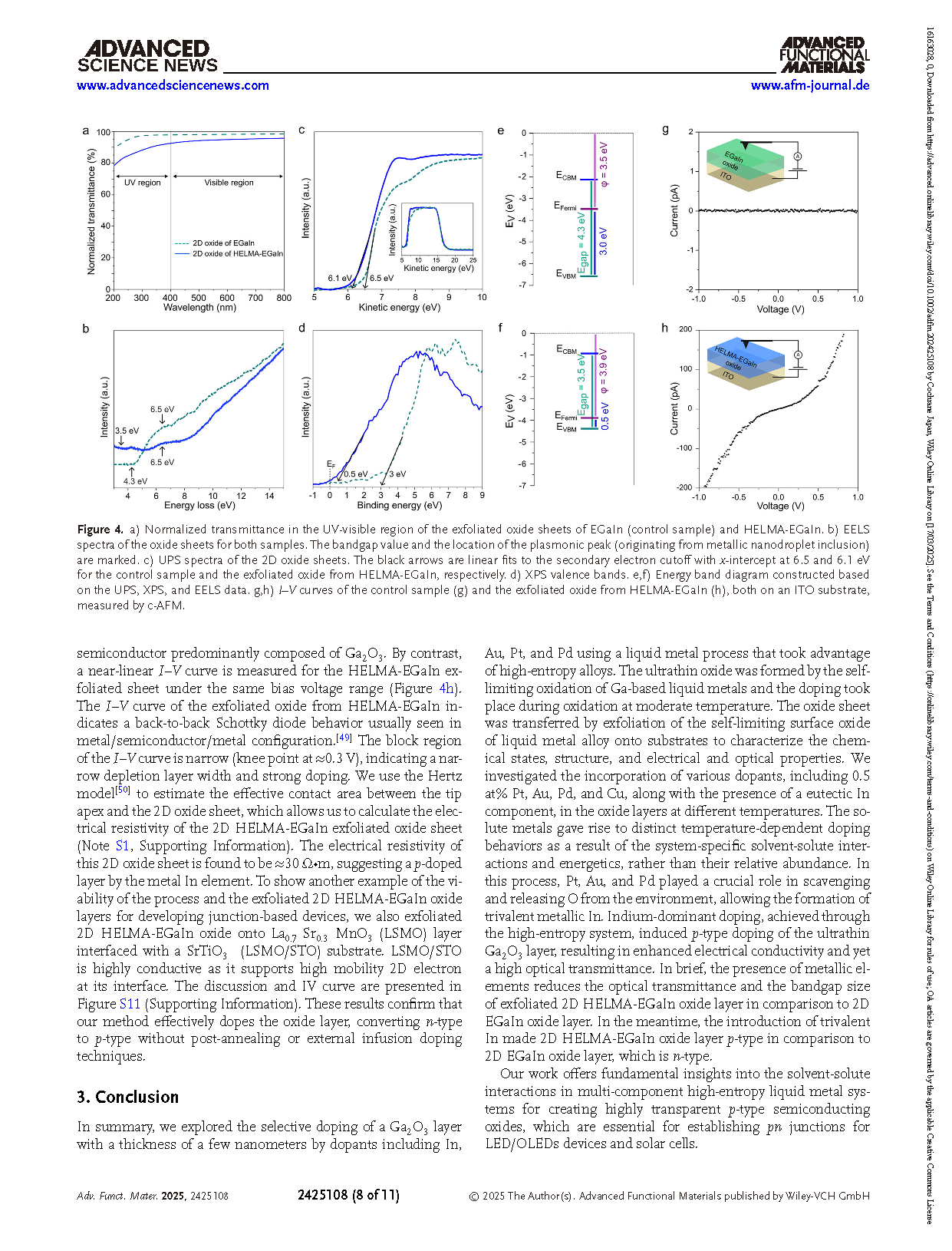
The naturally self-limiting oxide formed on the surface of liquid metals can be exfoliated and transferred onto various substrates. This oxide layer with a thickness of a few nanometers is typically highly transparent and can be engineered for applications in large-area optoelectronics. While the incorporation of solvated elements into the interfacial oxide of post-transition metal-based liquid metals is demonstrated for n-doping, achieving p-doping in such ultrathin oxide layers remains a significant challenge. In this study, the use of dissolved indium (In), platinum (Pt), gold (Au), palladium (Pd), and copper (Cu) in gallium (Ga)-based alloys is investigated to create a high-entropy liquid metal system. This allows the exfoliation of a p-doped ultrathin oxide layer, predominantly composed of gallium oxide (Ga2O3). The incorporation of these post-transition metals in this high-entropy system results in their atomic dispersion, with Cu exhibiting limited surface presence. The atomically dispersed Pt, Au, and Pd metals scavenge oxygen during exfoliation at moderate temperatures and release them during cooling down, promoting the emergence of trivalent metallic In in Ga oxide layer. This work presents a novel doping strategy at moderate temperatures to achieve p-doped liquid-metal-derived ultrathin Ga2O3 layers, which maintain high transparency.
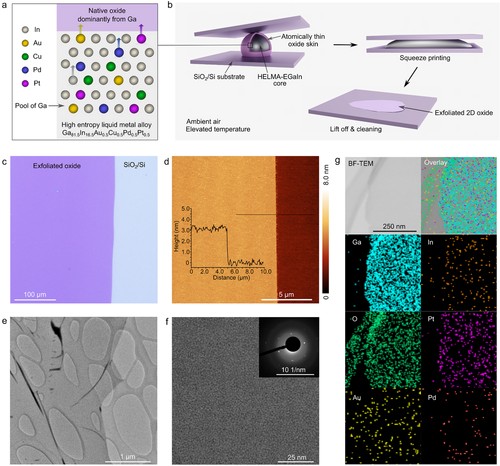
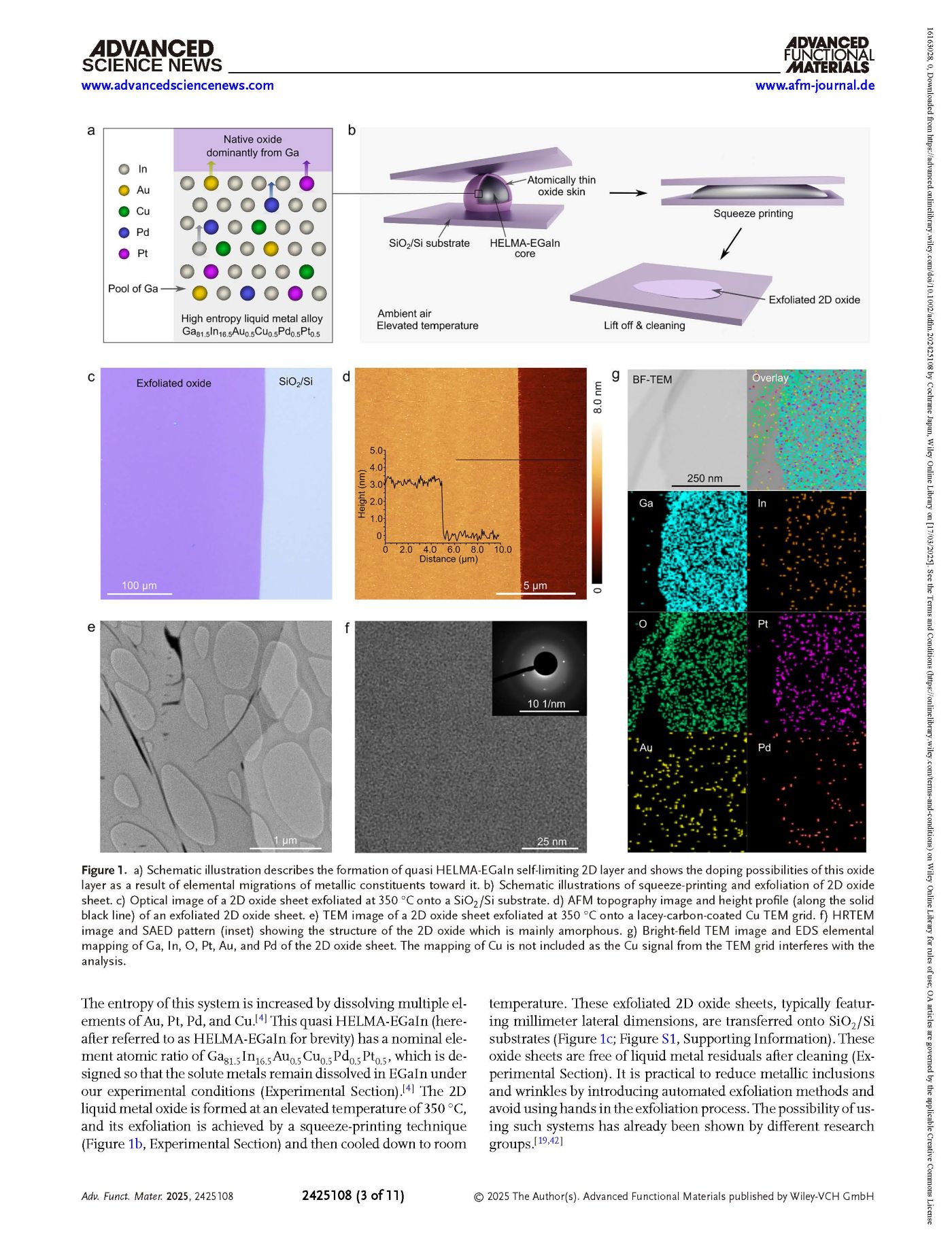
Figure 1. a) Schematic illustration describes the formation of quasi HELMA-EGaIn self-limiting 2D layer and shows the doping possibilities of this oxide layer as a result of elemental migrations of metallic constituents toward it. b) Schematic illustrations of squeeze-printing and exfoliation of 2D oxide sheet. c) Optical image of a 2D oxide sheet exfoliated at 350 °C onto a SiO2/Si substrate. d) AFM topography image and height profile (along the solid black line) of an exfoliated 2D oxide sheet. e) TEM image of a 2D oxide sheet exfoliated at 350 °C onto a lacey-carbon-coated Cu TEM grid. f) HRTEM image and SAED pattern (inset) showing the structure of the 2D oxide which is mainly amorphous. g) Bright-field TEM image and EDS elemental mapping of Ga, In, O, Pt, Au, and Pd of the 2D oxide sheet. The mapping of Cu is not included as the Cu signal from the TEM grid interferes with the analysis.
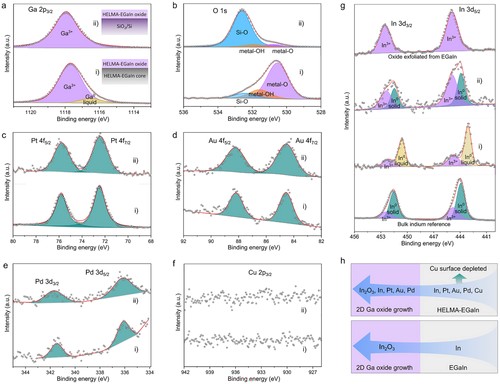
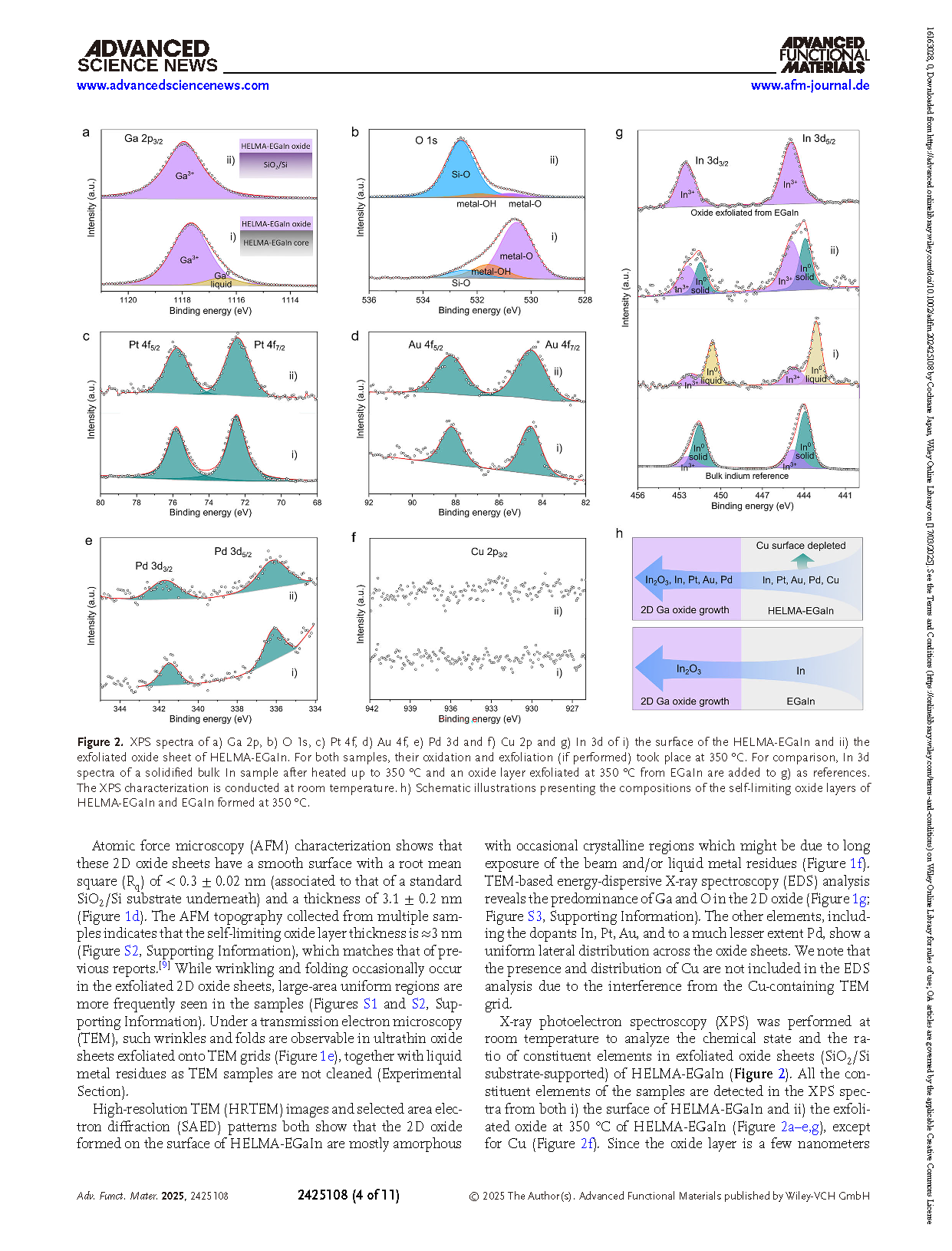
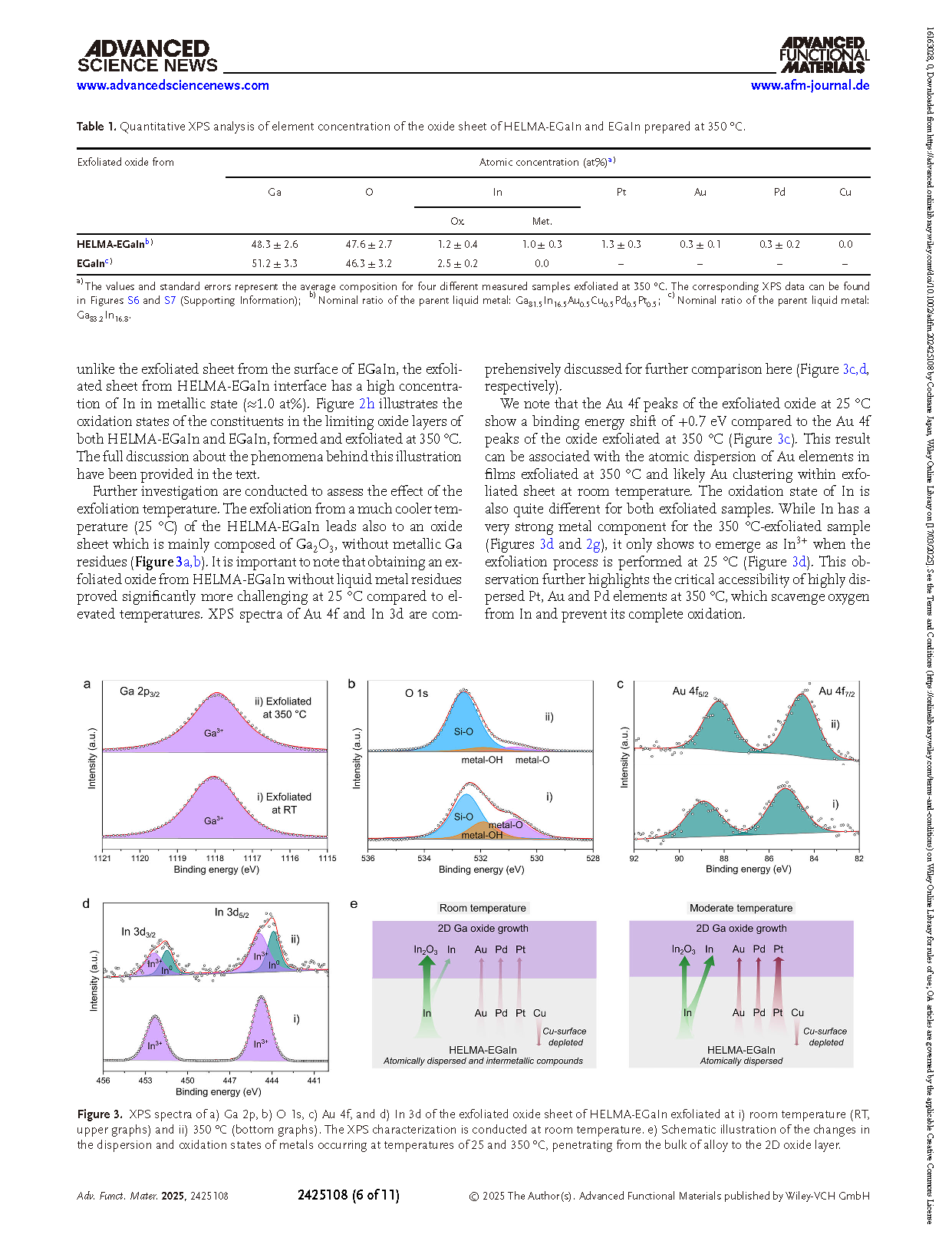
Figure 2. XPS spectra of a) Ga 2p, b) O 1s, c) Pt 4f, d) Au 4f, e) Pd 3d and f) Cu 2p and g) In 3d of i) the surface of the HELMA-EGaIn and ii) the exfoliated oxide sheet of HELMA-EGaIn. For both samples, their oxidation and exfoliation (if performed) took place at 350 °C. For comparison, In 3d spectra of a solidified bulk In sample after heated up to 350 °C and an oxide layer exfoliated at 350 °C from EGaIn are added to g) as references. The XPS characterization is conducted at room temperature. h) Schematic illustrations presenting the compositions of the self-limiting oxide layers of HELMA-EGaIn and EGaIn formed at 350 °C.
DOI:
doi.org/10.1002/adfm.202425108