【Member Papers】High-open-circuit voltage diamond alpha-voltaic battery with interface reconstructed by amorphous gallium oxide
日期:2026-01-19阅读:81
Nuclear batteries, with their miniature size, ability to withstand extreme environments, long operational lifetime, and excellent environmental adaptability, exhibit broad application prospects in deep space exploration, ocean and polar environments, desert regions, and biomedical fields. Diamond, as an ultra-wide-bandgap semiconductor, possesses outstanding radiation resistance and an exceptionally wide bandgap, making it an ideal material for α-voltaic batteries due to its potential to establish a high built-in potential barrier and to suppress radiation-induced damage. However, addressing interface leakage currents and low open-circuit voltage caused by Shockley–Read–Hall (SRH) recombination at oxygen-terminated diamond Schottky junction interfaces remains a major challenge.
In this work, we fabricated a nuclear battery in which the diamond Schottky contact interface is reconstructed by amorphous Gallium Oxide. Using a 241Am α source with an activity of 8.85 μCi/cm², a high open-circuit voltage of 2.41 V was achieved. In addition, a short-circuit current density of 6.6 nA/cm² and a maximum output power density of 9.72 nW/cm² were obtained, resulting in an overall α-voltaic energy conversion efficiency of 3.7%. The process reproducibility and temperature stability of the nuclear battery were also experimentally verified.
Further analysis of the interfacial energy band structure between diamond and amorphous Gallium Oxide revealed the relationship between carrier extraction and interface passivation. Based on an investigation of the chemical bonding states at the interface, variations in the concentrations of interfacial ketone and ester bonds were identified, along with their contributions to the uniformity of the electron potential barrier. On this basis, a new mechanism for amorphous Gallium Oxide–induced reconstruction of oxygen-terminated diamond interfaces is proposed. This work provides a new strategy for improving interfacial carrier transport in diamond and enhancing the open-circuit voltage of α-voltaic batteries.
The related results were published in the journal Carbon, a leading journal in carbon materials science and technology, under the title “High-open-circuit voltage diamond alpha-voltaic battery with interface reconstructed by amorphous gallium oxide.” The first author of the paper is doctoral student Chuanlong Li from the School of Astronautics, Harbin Institute of Technology, and the corresponding author is Associate Professor Benjian Liu from the same institution.
Background
With the expansion of exploration in extreme environments and the rapid development of micro-electromechanical systems (MEMS), the demand for long-lifetime, high–energy-density power sources is steadily increasing. Over the prolonged evolution of various power technologies—such as chemical batteries, photovoltaic devices, and supercapacitors—researchers have found that isotope batteries based on the radiovoltaic effect uniquely combine long service life, high energy density, miniature size, and excellent stability under extreme conditions. These advantages enable them to overcome many inherent limitations of conventional power sources, including the low energy density and poor low-temperature performance of chemical batteries, the environmental dependence of photovoltaic devices, and the fact that supercapacitors can store but not generate energy.
As an emerging class of radiovoltaic isotope batteries, however, it must be acknowledged that their power density and energy conversion efficiency have not yet reached their theoretical limits. Addressing this gap involves multidisciplinary challenges, including advances in semiconductor device architecture and materials preparation.
Diamond, owing to its wide bandgap of 5.45 eV at room temperature, high displacement energy, high critical electric field strength, high electron and hole mobilities, and exceptional thermal conductivity, is an ideal material for high-efficiency isotope batteries, power devices, and ultraviolet detectors. In particular, the combination of high displacement energy and wide bandgap endows diamond with outstanding tolerance to extreme environments such as high/low temperatures and intense radiation, making it one of the most promising materials for isotope battery applications. In principle, once challenges related to diamond doping, epitaxial growth, and precision processing are resolved, its performance could surpass current technologies by a qualitative margin. However, efficient n-type doping of diamond remains unresolved, and the widely adopted Schottky barrier structures still suffer from poor interfacial contact, barrier inhomogeneity, and high carrier recombination rates. These issues severely limit the open-circuit voltage and conversion efficiency of diamond-based Schottky isotope batteries, keeping their performance far below theoretical expectations.
In this study, we propose a new strategy to enhance the open-circuit voltage of isotope batteries through amorphous Gallium Oxide (AGO)–induced reconstruction of oxygen-terminated diamond interfaces. By employing a staggered band alignment, non-equilibrium carriers at the Schottky contact interface can be selectively extracted and passivated, thereby reducing the recombination probability of non-equilibrium carriers at interfacial defect states. Enhanced carrier collection efficiency consequently leads to improved isotope battery performance.
Further investigation of interfacial bonding reveals that, due to conjugation effects, carbon–oxygen atoms in ketone bonds cause electron cloud accumulation at the oxygen termination, forming a high potential barrier for electrons near the interface. This barrier hinders the transport of electrons collected near the cathode into the Schottky electrode material, while simultaneously attracting non-equilibrium holes and inducing significant recombination losses. In this work, gallium oxide thin films prepared by radio-frequency magnetron sputtering reconstruct the contact interface via the action of reduced gallium ions. This process reduces the density of ketone groups while introducing a higher proportion of ester groups, thereby mitigating electron cloud accumulation caused by electronegativity differences. As a result, the interfacial electron barrier is lowered, carrier transport across the interface is improved, and an open-circuit voltage exceeding 2.41 V is achieved for a single-junction diamond isotope battery, with the conversion efficiency increased to 3.7%. Reproducibility tests and temperature-dependent measurements further confirm the feasibility of the process and its thermal stability.
Overall, this work provides a new approach for improving diamond Schottky contact interfaces and enhancing the performance of diamond-based isotope batteries.
Highlights
1、An ultrathin amorphous Gallium Oxide dielectric layer was fabricated by RF magnetron sputtering, achieving a dark leakage current as low as 0.1 pA. Under 241Am irradiation with an activity of 8.85 μCi/cm², the single-junction battery exhibits an open-circuit voltage of 2.41 V, a short-circuit current density of 6.6 nA/cm², a power density of 9.72 nW/cm², and a conversion efficiency of up to 3.7%.
2、XPS and KPFM measurements were used to determine the band alignment at the interface between oxygen-terminated diamond and amorphous Gallium Oxide films, confirming the roles of electron extraction and hole passivation near the interface and reducing recombination losses of non-equilibrium carriers.
3、Interfacial bonding analysis reveals the barrier effects of ketone and ester groups at oxygen-terminated diamond interfaces. By reducing electron delocalization, interfacial carrier transport is improved and recombination is suppressed, elucidating the mechanism by which interface states influence isotope battery performance.
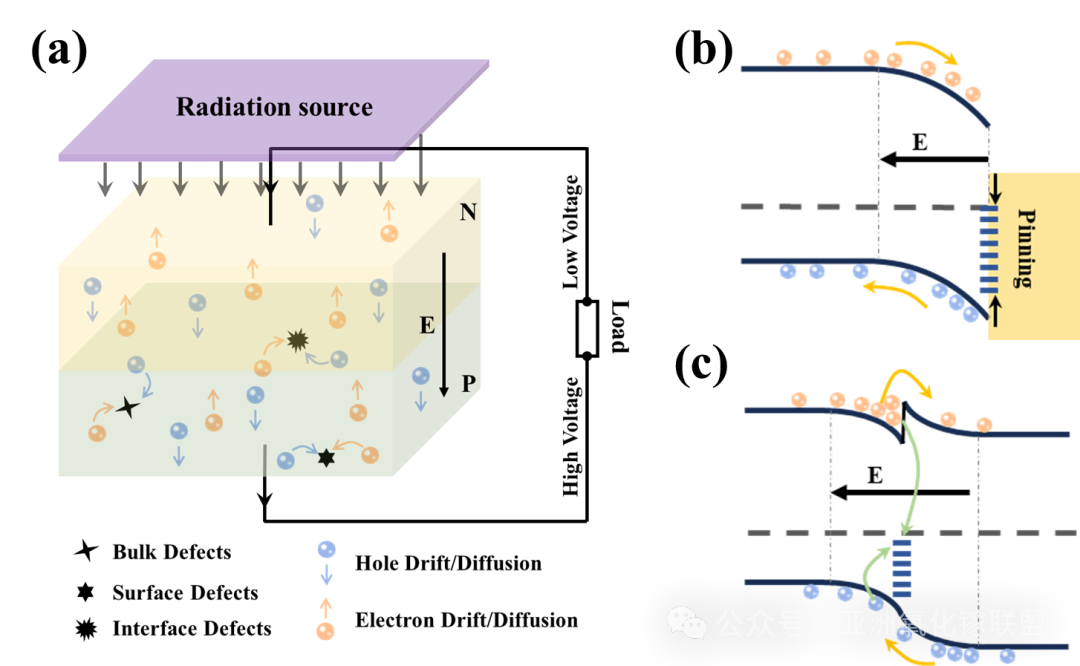
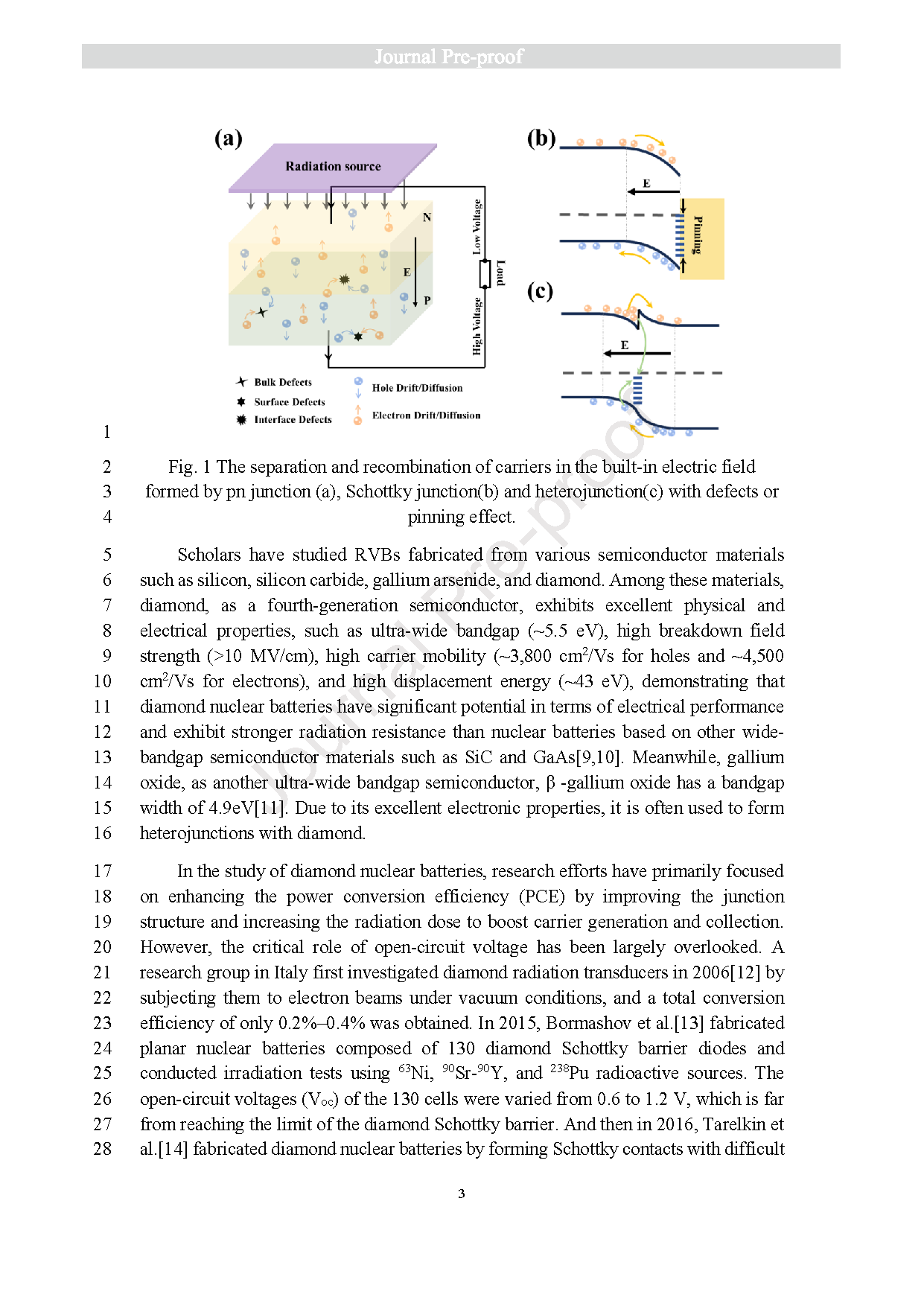
Fig. 1 The separation and recombination of carriers in the built-in electric field 3 formed by pn junction (a), Schottky junction(b) and heterojunction(c) with defects or pinning effect
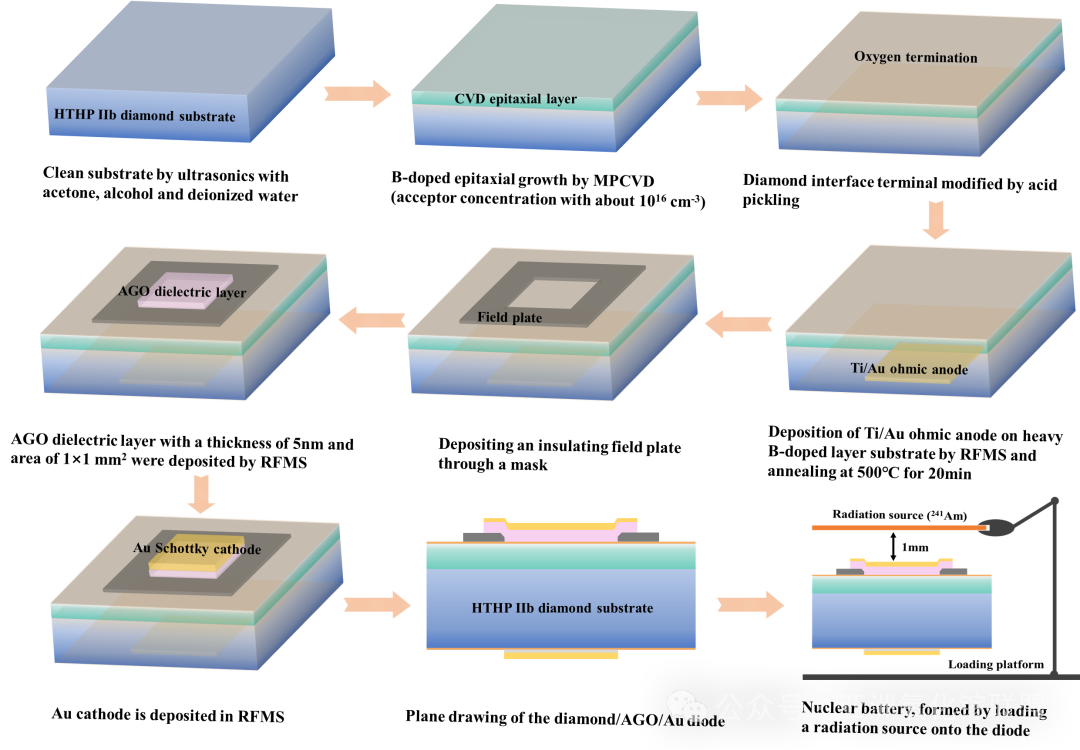
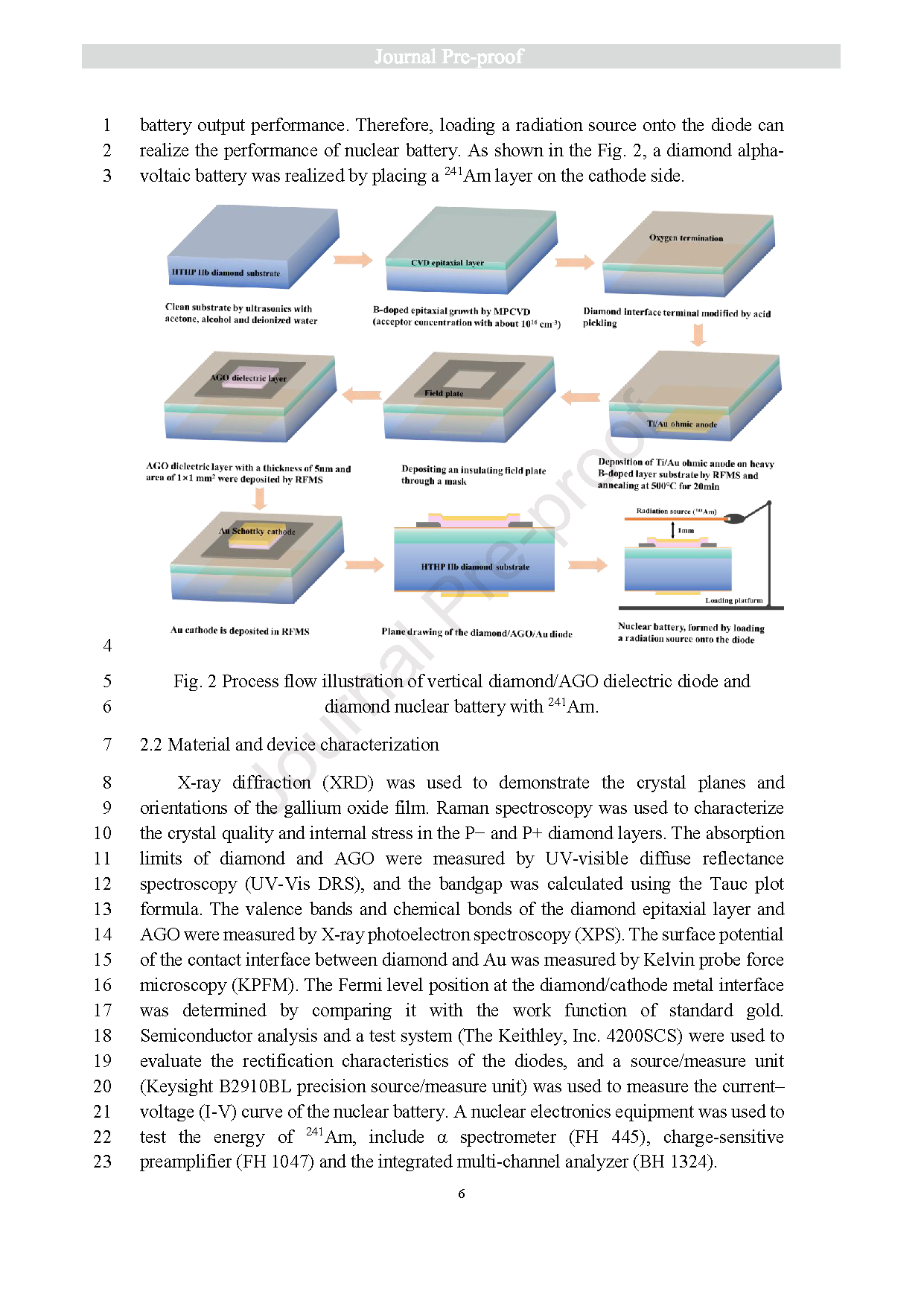
Fig. 2 Process flow illustration of vertical diamond/AGO dielectric diode and diamond nuclear battery with 241 Am.
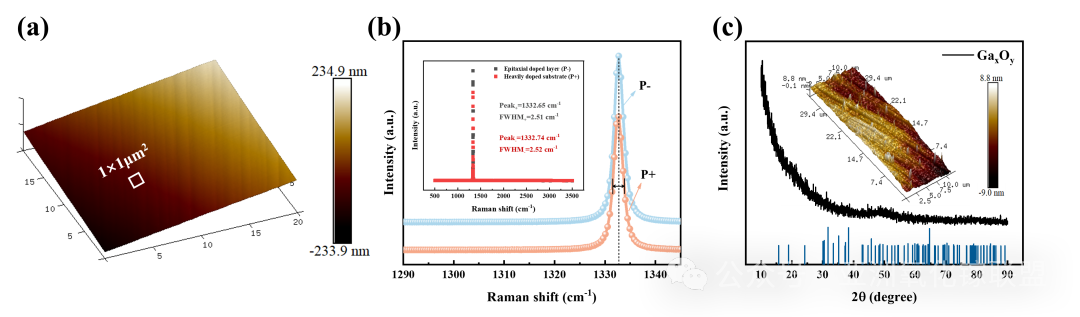
Fig. 3 (a) AFM scanning of P- layer (the selection range is white). (b) Raman response of P+ layer and P- layer. (c) XRD scanning of AGO films (The blue line represents the standard card data of β - Ga2O3)
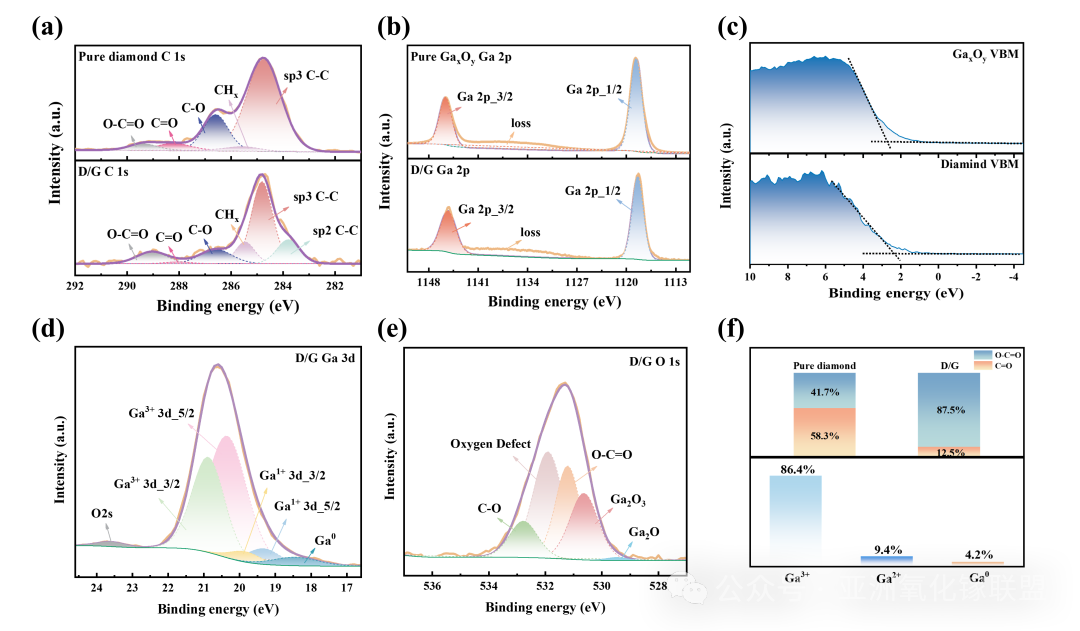
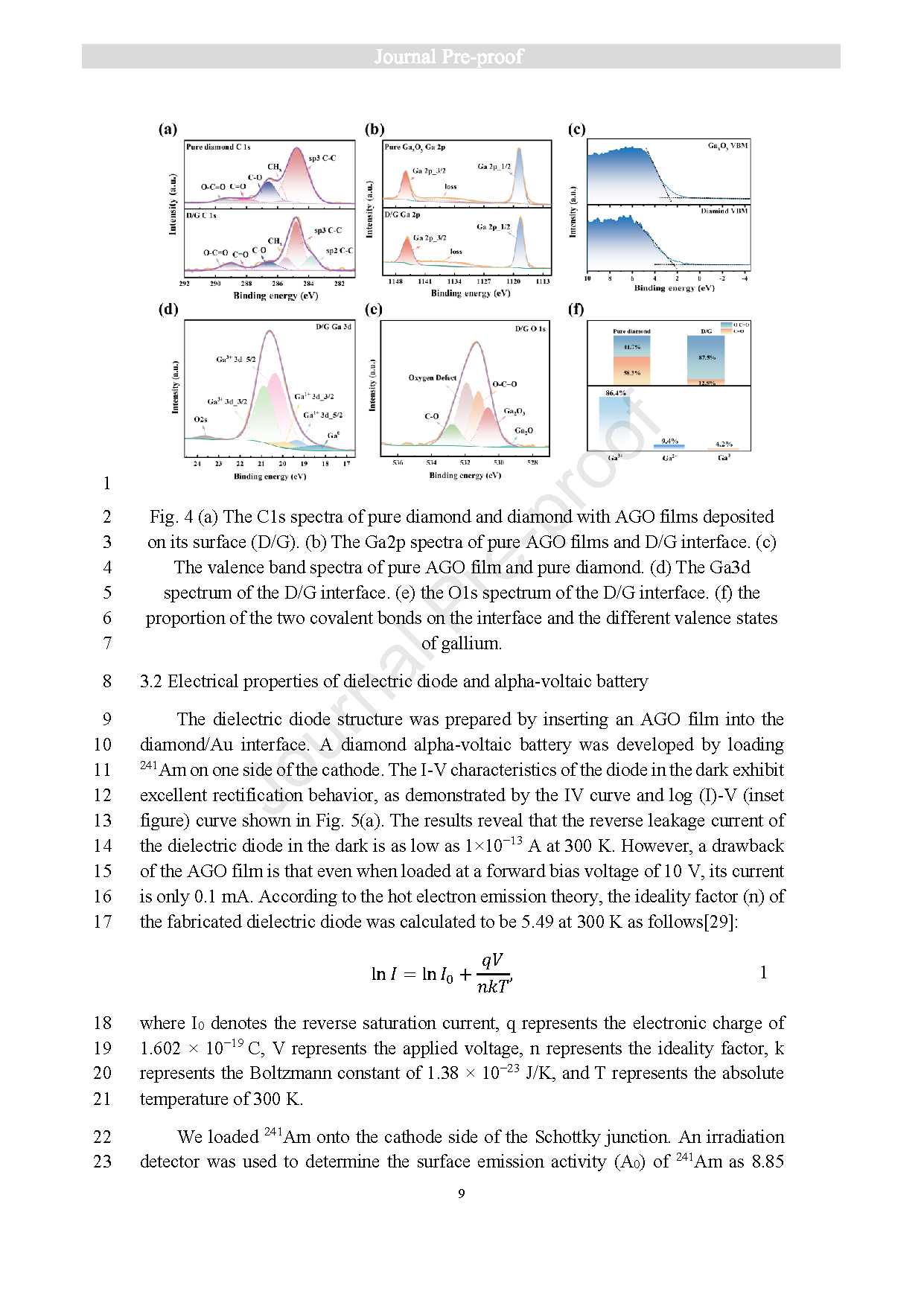
Fig. 4 (a) The C1s spectra of pure diamond and diamond with AGO films deposited on its surface (D/G). (b) The Ga2p spectra of pure AGO films and D/G interface. (c) The valence band spectra of pure AGO film and pure diamond. (d) The Ga3d spectrum of the D/G interface. (e) the O1s spectrum of the D/G interface. (f) the proportion of the two covalent bonds on the interface and the different valence states of gallium.
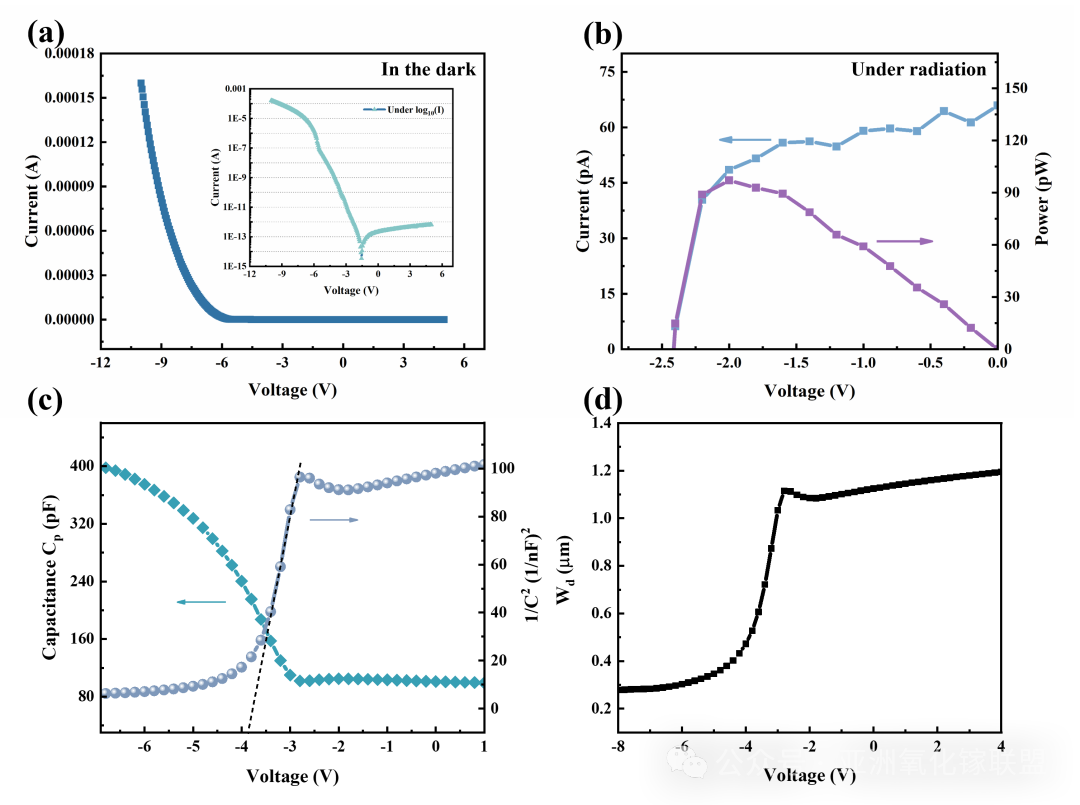
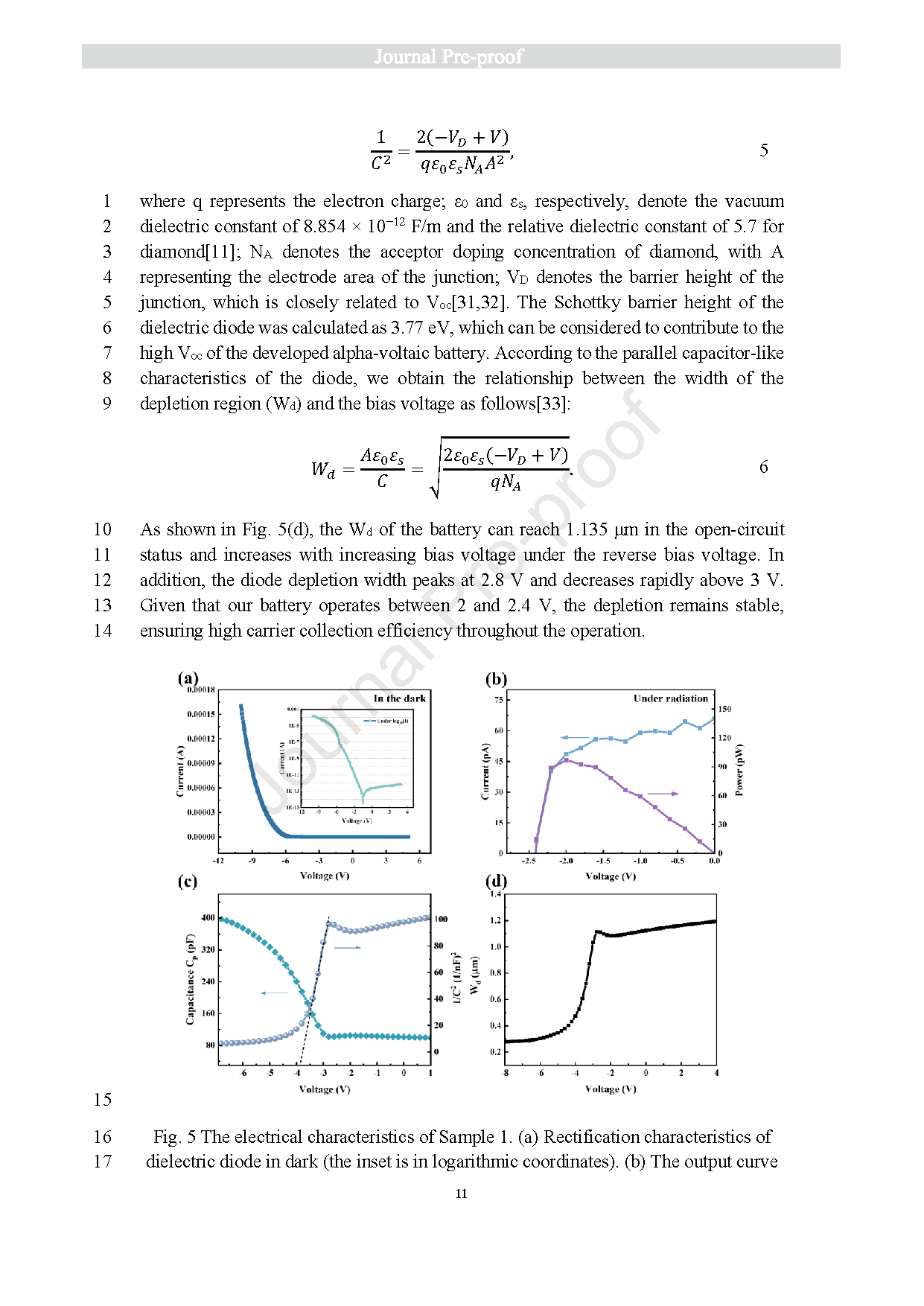
Fig. 5 The electrical characteristics of Sample 1. (a) Rectification characteristics of dielectric diode in dark (the inset is in logarithmic coordinates). (b) The output curve of nuclear battery under radiation (241Am). (c) The C-V/1/C2 1 -V curve of dielectric diode. (d) The relationship between depletion width and bias voltage.
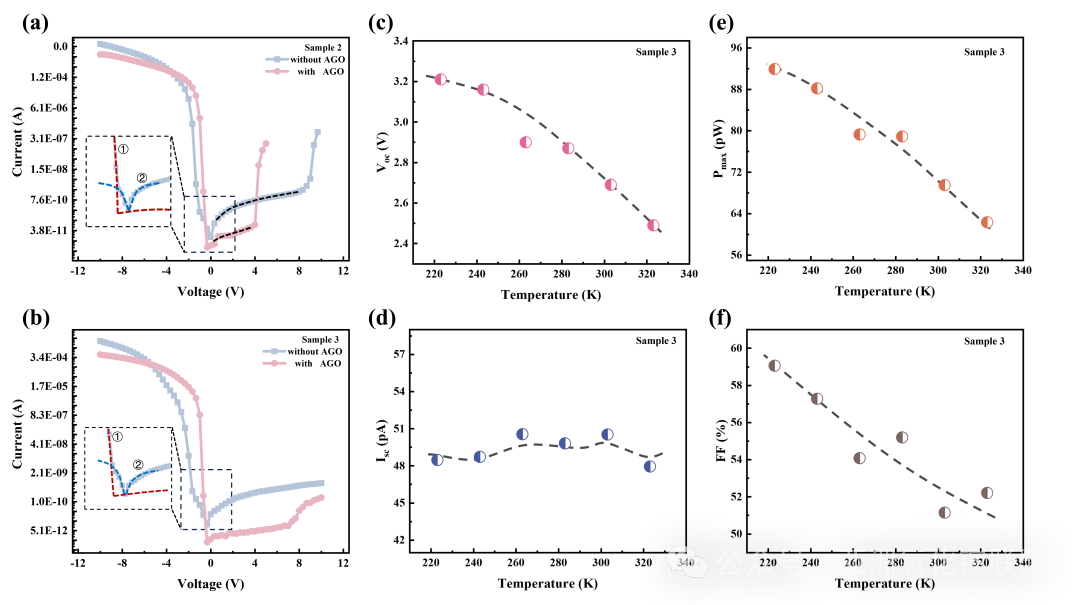
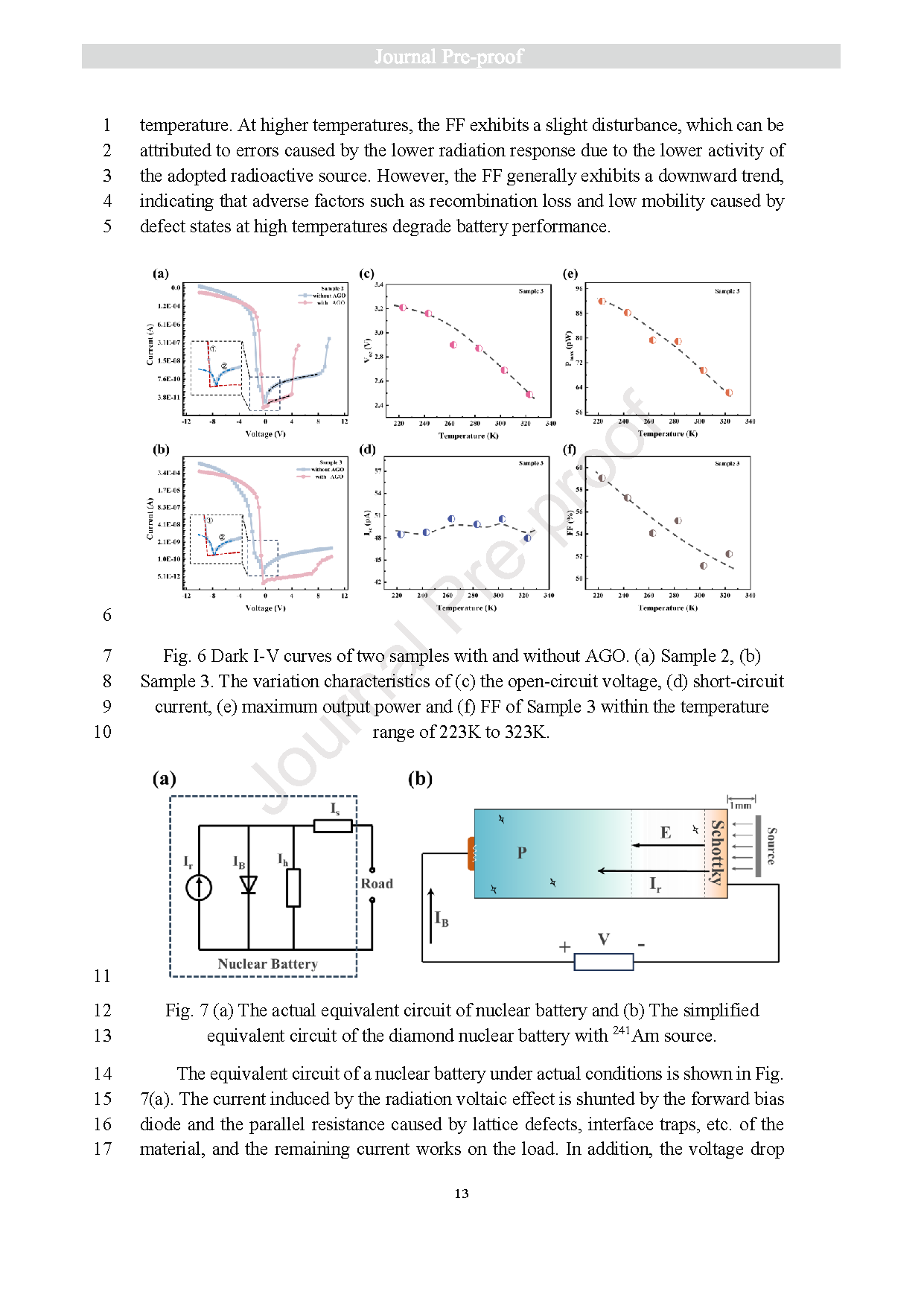
Fig. 6 Dark I-V curves of two samples with and without AGO. (a) Sample 2, (b) Sample 3. The variation characteristics of (c) the open-circuit voltage, (d) short-circuitcurrent, (e) maximum output power and (f) FF of Sample 3 within the temperature range of 223K to 323K.
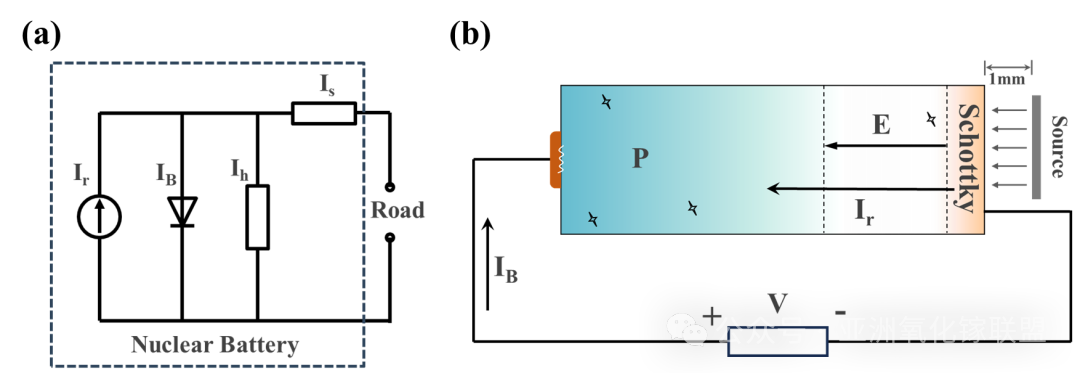
Fig. 7 (a) The actual equivalent circuit of nuclear battery and (b) The simplified equivalent circuit of the diamond nuclear battery with 241 13 Am source.
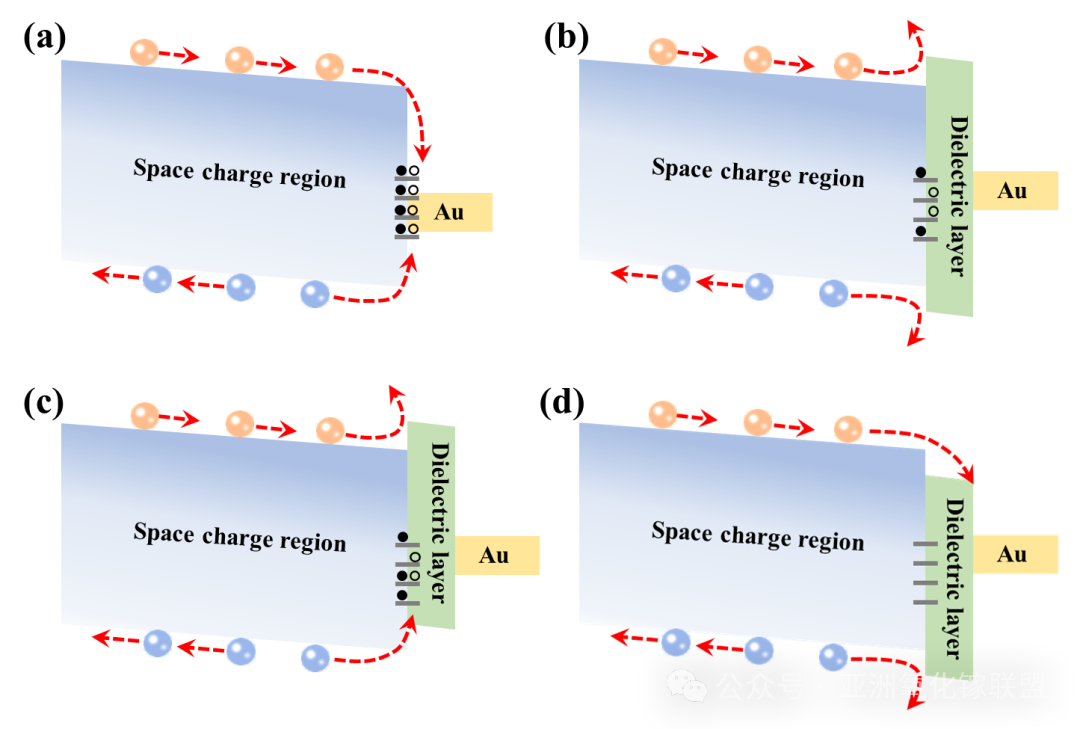
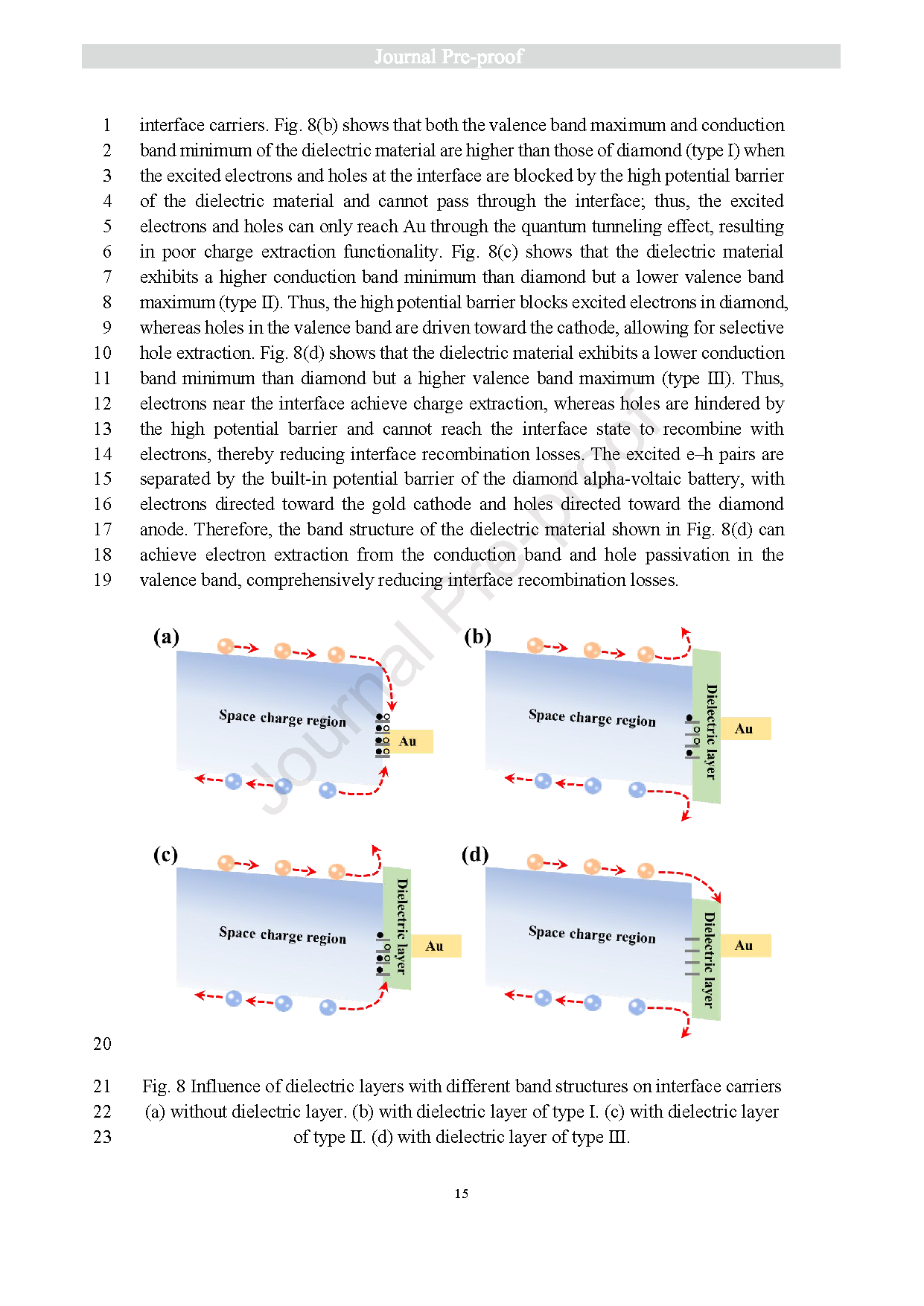
Fig. 8 Influence of dielectric layers with different band structures on interface carriers (a) without dielectric layer. (b) with dielectric layer of type Ⅰ. (c) with dielectric layerof type Ⅱ. (d) with dielectric layer of type Ⅲ.
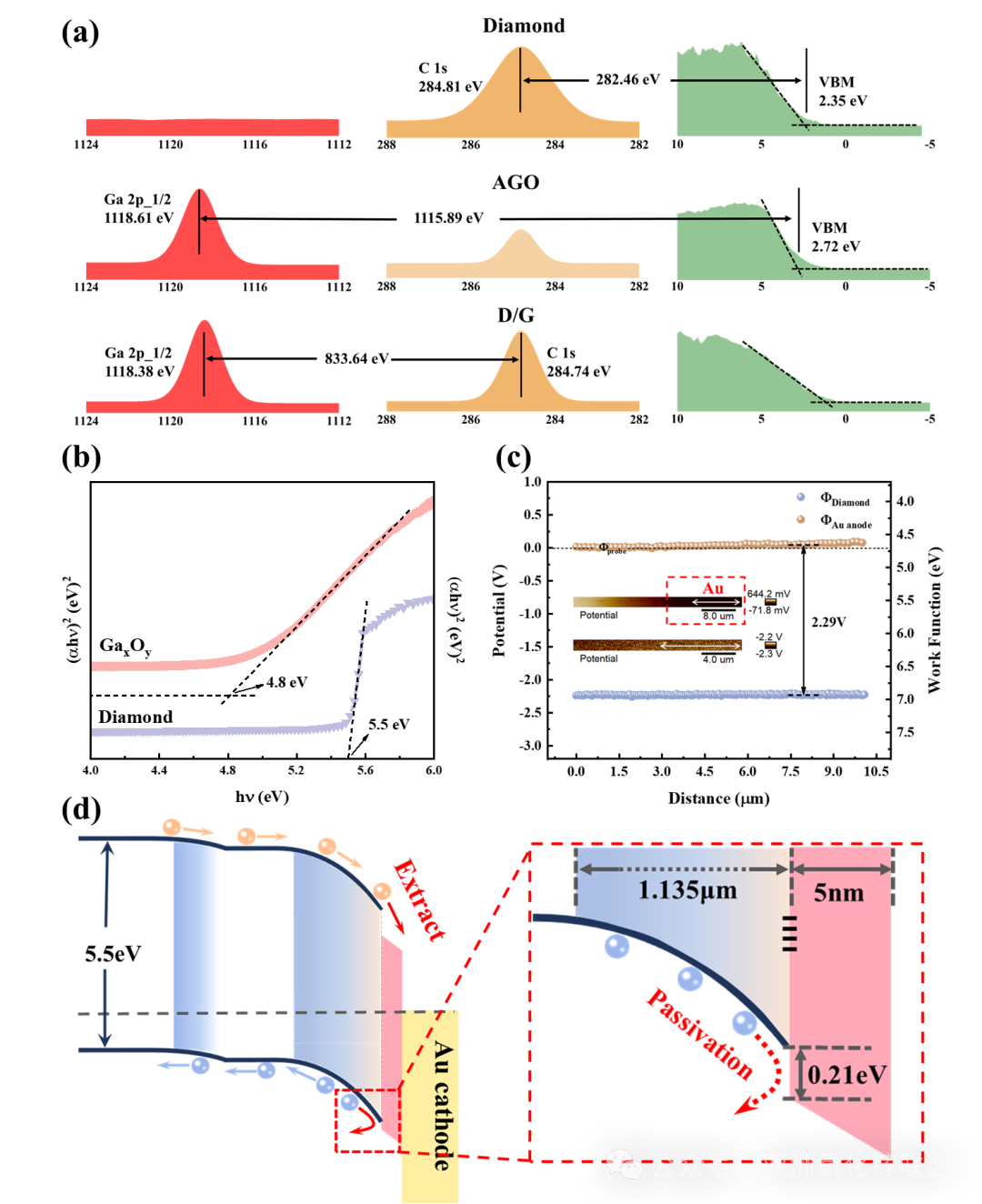
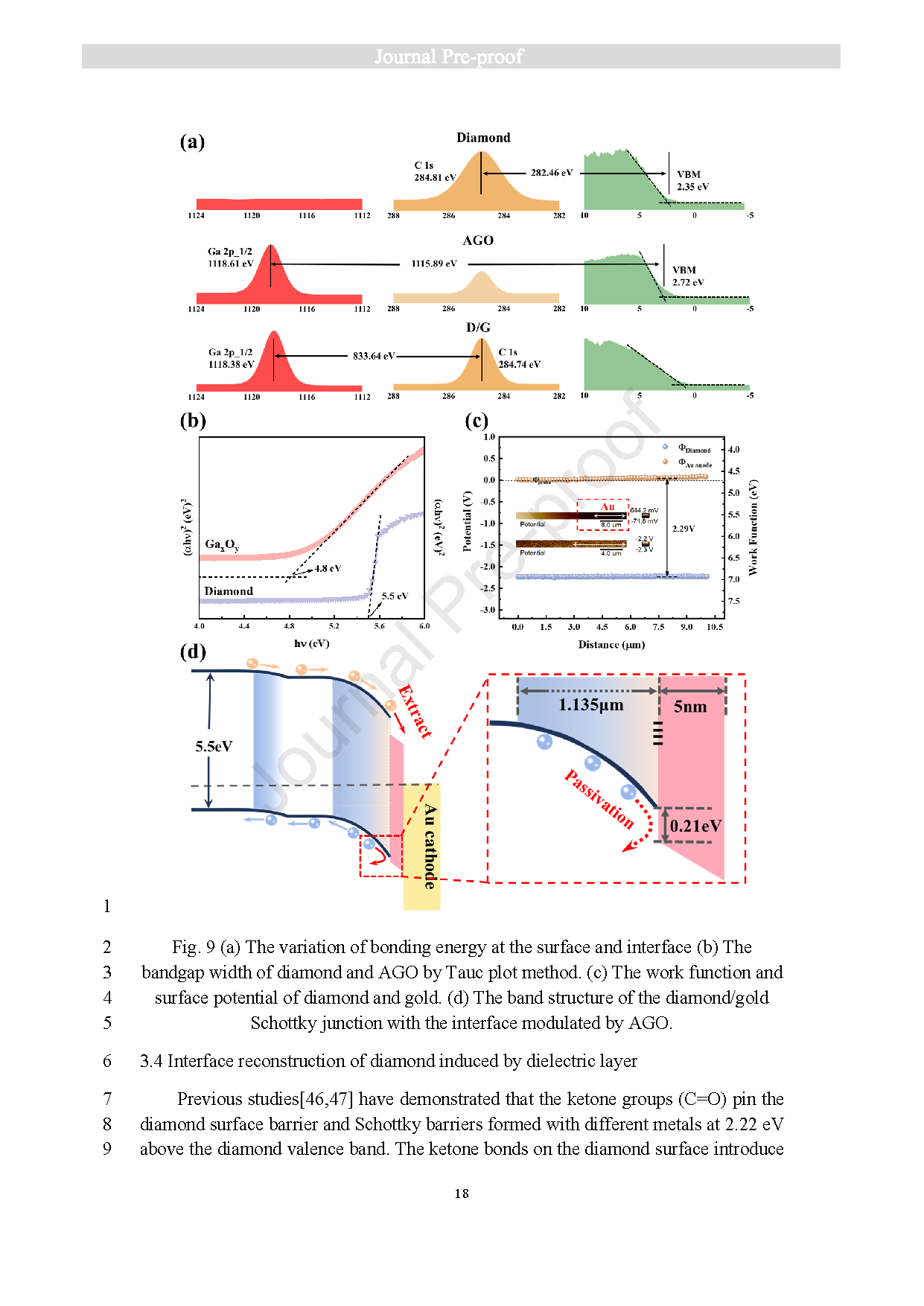
Fig. 9 (a) The variation of bonding energy at the surface and interface (b) The bandgap width of diamond and AGO by Tauc plot method. (c) The work function andsurface potential of diamond and gold. (d) The band structure of the diamond/gold Schottky junction with the interface modulated by AGO.
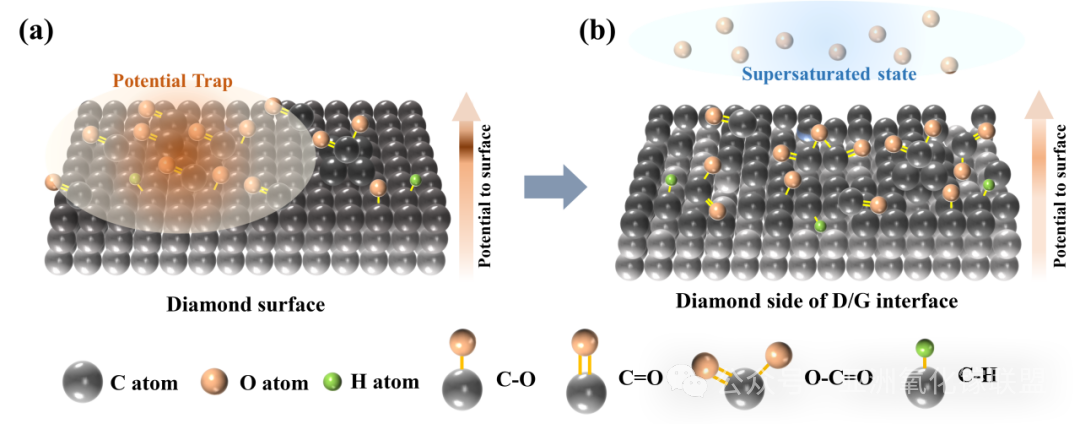
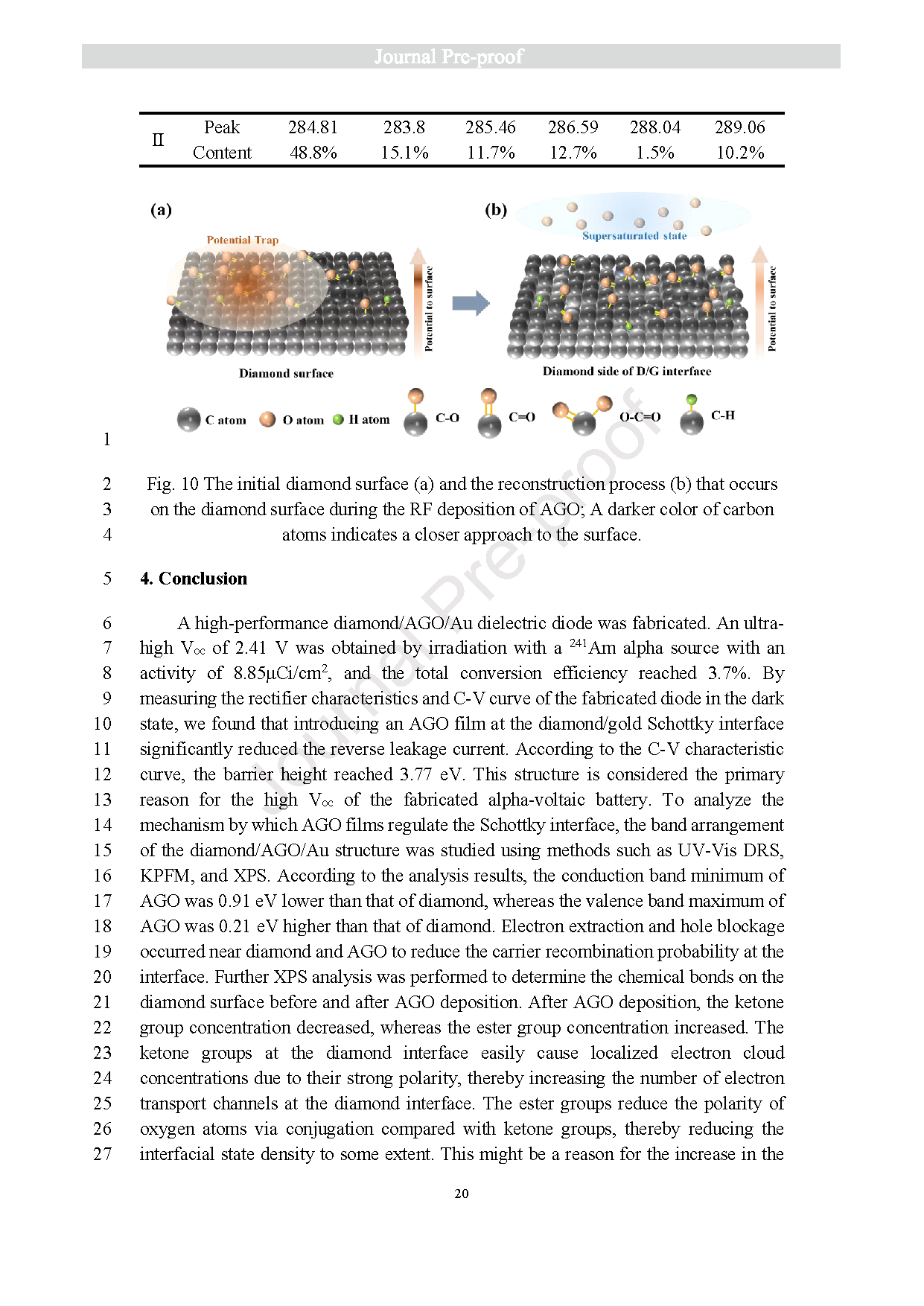
Fig. 10 The initial diamond surface (a) and the reconstruction process (b) that occurs on the diamond surface during the RF deposition of AGO; A darker color of carbon atoms indicates a closer approach to the surface.
Summary and Outlook
This study proposes a new mechanism for enhancing the open-circuit voltage of alpha-voltaic batteries through amorphous gallium oxide (AGO)-induced reconstruction of the oxygen-terminated diamond interface. By exploiting the band alignment at the AGO–oxygen-terminated diamond interface, non-equilibrium carriers at the interface are efficiently extracted and passivated, effectively reducing interfacial recombination. The study further reveals the impact of ketone and ester groups on the interfacial potential. The use of reduced-state gallium ions via radio-frequency magnetron sputtering decreases the ketone concentration, while the increased ester content reduces the high interfacial electron barrier caused by electron delocalization, thereby improving carrier transport across the interface and enhancing the overall output performance of diamond-based alpha-voltaic batteries.
C. Li, B. Liu, B. Liang, Z. Chen, W. Zhang, W. Liang, Y. Zuo, H. Jia, T. Chen, Z. Sang, L. Liu, K. Liu, D. Lu, A.P. Bolshakov, V.G. Ralchenko, B. Dai, J. Zhu, High-open-circuit voltage diamond alpha-voltaic battery with interface reconstructed by amorphous gallium oxide, Carbon. 10.1016/j.carbon.2026.121251.