【Domestic Papers】Interface Hydrophobic Engineering on Copper Species-Modified Ga₂O₃ Nanofibers for Enhanced Nitrate Electroreduction
日期:2026-01-19阅读:86
Researchers from the Donghua University have published a dissertation titled "Interface Hydrophobic Engineering on Copper Species-Modified Ga2O3 Nanofibers for Enhanced Nitrate Electroreduction" in Advanced Functional Materials.
Project Support
This work was supported by the Fundamental Research Funds for the Central Universities (No. 2232025A-07), the National Natural Science Foundation of China (No. 52503378), the Chenguang Program of Shanghai Education Development Foundation and Shanghai Municipal Education Commission (No. 24CGA66), the Program of Shanghai Academic Research Leader (No. 23XD1400100), and the Natural Science Foundation of Shanghai (No. 23ZR1401400).
Background
The nitrogen cycle plays a critical role in maintaining ecological balance; however, intensive human activities, such as excessive fertilizer use and industrial emissions, have severely disrupted this cycle, leading to nitrate accumulation in aquatic environments and posing significant risks to ecosystems and public health. Conventional nitrate removal technologies suffer from inherent limitations, including sluggish kinetics, high energy consumption, and the generation of undesirable by-products. In contrast, electrocatalytic nitrate reduction to ammonia (NO₃RR-to-NH₃) offers a promising and sustainable alternative by simultaneously achieving nitrate remediation and producing ammonia, a valuable carbon-free energy carrier and chemical feedstock.
Despite its advantages, NO₃RR is a complex multielectron–multiproton process, and its practical application is hindered by the difficulty of developing electrocatalysts with simultaneously high activity, selectivity, and long-term stability. This challenge becomes particularly pronounced under low nitrate concentrations, where the competing hydrogen evolution reaction (HER) significantly suppresses Faradaic efficiency. Solely relying on intrinsic catalyst structure optimization is often insufficient to balance HER suppression and NO₃RR enhancement, prompting increasing interest in interface engineering strategies to regulate the reaction microenvironment.
In this work, a dual synergistic strategy integrating electronic structure modulation and interfacial microenvironment regulation is proposed. Copper species and oxygen vacancies are in situ generated on Ga₂O₃ nanofibers via hydrogen thermal reduction, effectively tuning the electronic structure and enhancing the activation of both nitrate species and adsorbed hydrogen intermediates (H*). Meanwhile, a low-surface-energy self-assembled monolayer is introduced to construct a hydrophobic interface, which regulates proton availability, suppresses HER, and promotes preferential nitrate adsorption. Benefiting from this cooperative design, the resulting r-Cu/Ga₂O₃-SAM catalyst delivers an exceptionally high ammonia yield rate and Faradaic efficiency under low nitrate concentrations, demonstrating significantly improved performance for electrocatalytic nitrate reduction to ammonia.
Abstract
Suppressing the competing hydrogen evolution reaction (HER) and inefficient mass transport remains a major obstacle for electrocatalytic nitrate reduction reactions (NO₃RR), particularly in low-nitrate-concentration industrial wastewater. Nevertheless, previous studies have primarily focused on optimizing intrinsic catalyst activity, yet achieving high selectivity and activity simultaneously remains challenging. Herein, we report a dual-coordination strategy to modulate the surface electronic structure and interfacial microenvironment of nanofiber catalysts, thereby enabling simultaneous HER suppression and enhanced electrocatalytic activation of nitrate (NO₃⁻).
Specifically, a reduced copper species–modified gallium oxide (r-Cu/Ga₂O₃) nanofiber catalyst with abundant Cu species and oxygen vacancies is obtained via thermal reduction of the oxide precursor through an in situ exsolution method, which significantly enhances nitrate adsorption and activation. Moreover, a low-surface-energy monolayer interface is assembled on the nanofiber surface to regulate the reaction microenvironment, restricting proton transfer to active sites and kinetically inhibiting HER.
As a result, the prepared r-Cu/Ga₂O₃–SAM nanofiber catalyst achieves an NH₃ yield of 22.36 mg h⁻¹ mg⁻¹ at −1.15 V and a Faradaic efficiency (FE) of 95.48% at −1.05 V versus the reversible hydrogen electrode (RHE) in a 20 mM NO₃⁻ solution. We expect that these findings will provide new insights and practical guidance for the rational design of efficient electrocatalytic NO₃RR catalysts under low NO₃⁻ concentration conditions.
Conclusion
To summarize, we have successfully achieved the concurrent enhancement of electrocatalytic nitrate reduction reactions (NO₃RR) and suppression of the competing hydrogen evolution reaction (HER) via a synergistic strategy that modulates both the electronic structure and interfacial microenvironment of the nanofiber catalyst. Specifically, r-Cu/Ga₂O₃ nanofibers were designed as efficient electrocatalysts for NO₃RR through in situ metal deposition enabled by thermal reduction of metal oxides. The optimized electronic states, arising from increased oxygen vacancies (Oᵥ) and enriched Cu species on the catalyst surface, serve as pivotal factors in facilitating electrocatalytic NO₃RR.
Furthermore, the construction of a low-surface-energy self-assembled monolayer (SAM) on the r-Cu/Ga₂O₃ nanofibers effectively alters the interfacial microenvironment, elevating the energy barrier for HER and thereby improving the Faradaic efficiency (FE) of NO₃RR. Benefiting from this synergistic strategy, the r-Cu/Ga₂O₃–SAM nanofiber catalyst delivers an NH₃ yield of 22.36 mg h⁻¹ mg⁻¹ at −1.15 V and achieves a FE of 95.48% at −1.05 V versus the reversible hydrogen electrode (RHE) in a 20 mM NO₃⁻ solution.
This study is expected to provide novel insights into the systematic design of both electronic structure and interfacial microenvironment of electrocatalysts for the efficient treatment of low-concentration industrial wastewater.
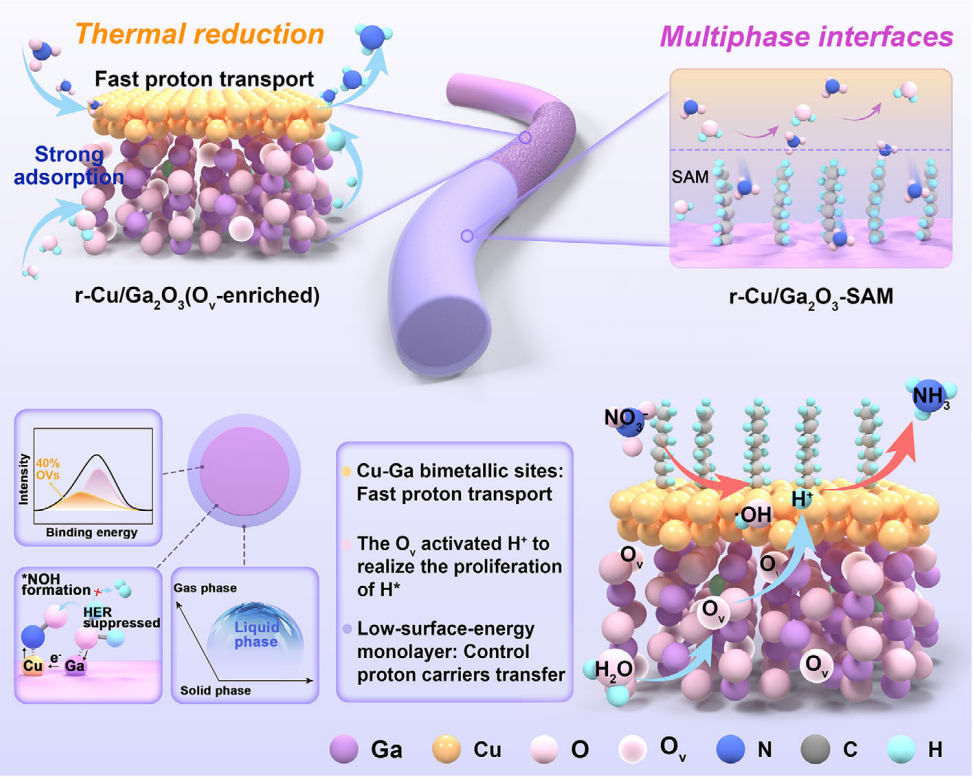
Figure 1. Schematic diagram illustrating the synthesis of r-Cu/Ga₂O₃ nanofibers via thermal reduction using the in situ exsolution method, and the subsequent assembly of a low-surface-energy self-assembled monolayer (SAM) on the r-Cu/Ga₂O₃ nanofibers. The SAM modulates the interfacial microenvironment, enhancing the electrocatalytic reduction of nitrate (NO₃⁻) reactions.

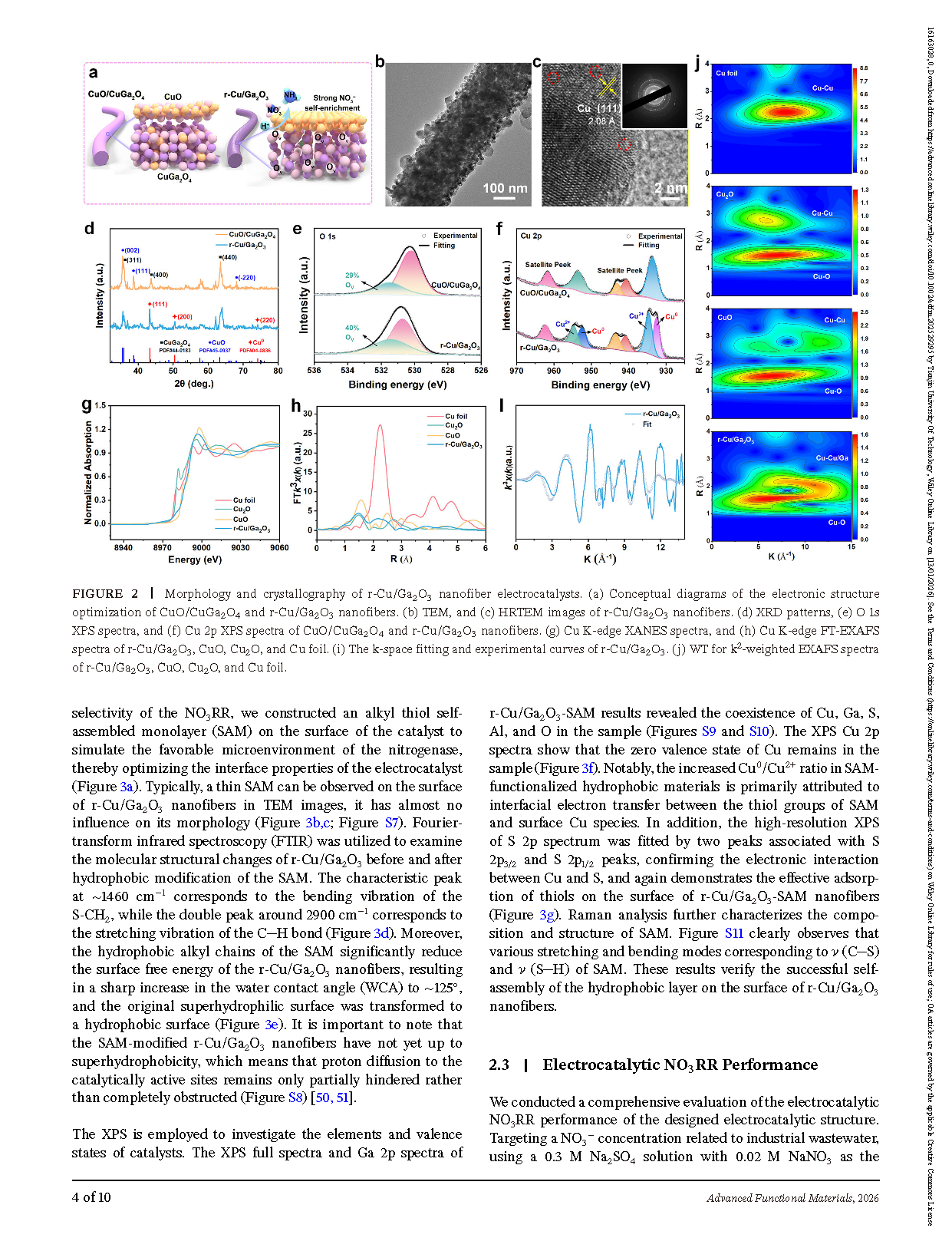
Figure 2. Morphology and crystallography of r-Cu/Ga₂O₃ nanofiber electrocatalysts. (a) Conceptual diagrams illustrating the electronic structure optimization of CuO/CuGa₂O₄ and r-Cu/Ga₂O₃ nanofibers. (b) TEM and (c) HRTEM images of r-Cu/Ga₂O₃ nanofibers. (d) XRD patterns, (e) O 1s XPS spectra, and (f) Cu 2p XPS spectra of CuO/CuGa₂O₄ and r-Cu/Ga₂O₃ nanofibers. (g) Cu K-edge XANES spectra, and (h) Cu K-edge FT-EXAFS spectra of r-Cu/Ga₂O₃, CuO, Cu₂O, and Cu foil. (i) k-space fitting and experimental curves of r-Cu/Ga₂O₃. (j) Wavelet transform (WT) for k²-weighted EXAFS spectra of r-Cu/Ga₂O₃, CuO, Cu₂O, and Cu foil.
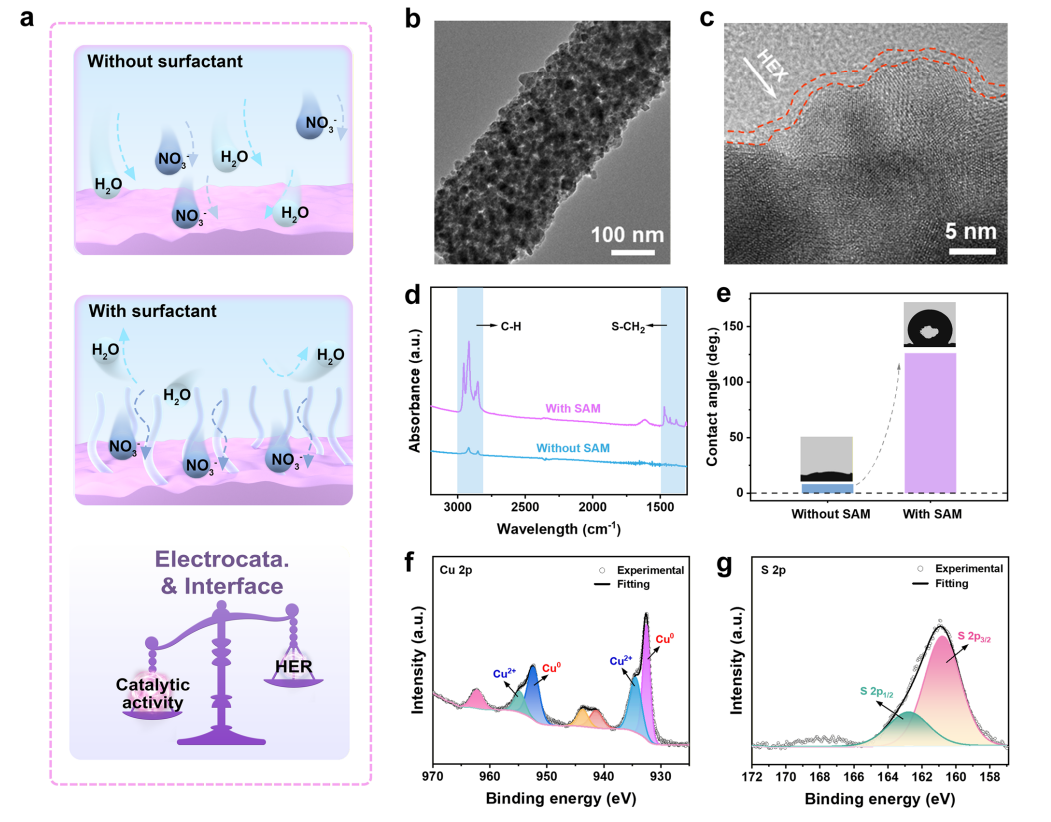
Figure 3. Interfacial microenvironment design of r-Cu/Ga₂O₃-based nanofiber electrocatalysts. (a) Schematic diagram illustrating the hydrophobic interface and microenvironment regulation. (b) TEM and (c) HRTEM images of r-Cu/Ga₂O₃-SAM nanofibers. Comparison of (d) normalized FTIR spectra and (e) contact angles (CA) between membranes composed of r-Cu/Ga₂O₃ and r-Cu/Ga₂O₃-SAM nanofibers. (f) Cu 2p and (g) S 2p XPS spectra of r-Cu/Ga₂O₃-SAM.
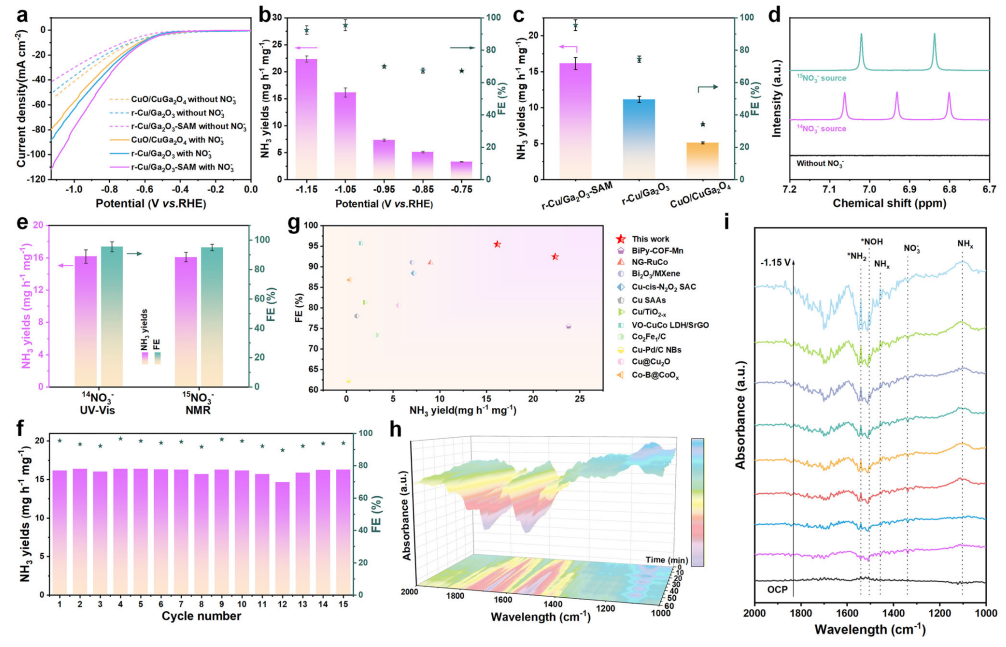
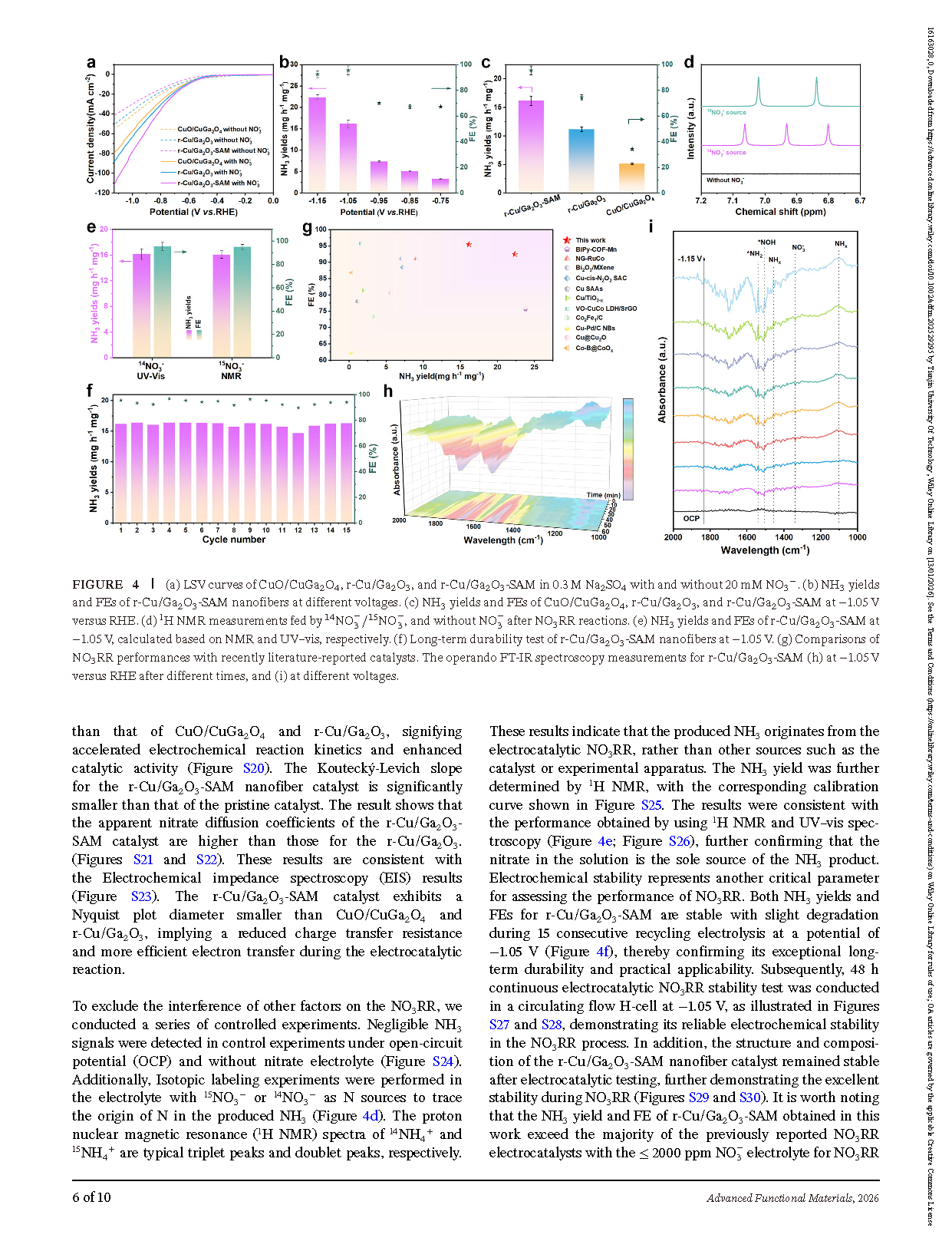
Figure 4. Electrocatalytic nitrate reduction performance of CuO/CuGa₂O₄, r-Cu/Ga₂O₃, and r-Cu/Ga₂O₃-SAM nanofibers.(a) LSV curves in 0.3 M Na₂SO₄ with and without 20 mM NO₃⁻. (b) NH₃ yields and Faradaic efficiencies (FEs) of r-Cu/Ga₂O₃-SAM nanofibers at different applied voltages. (c) NH₃ yields and FEs of CuO/CuGa₂O₄, r-Cu/Ga₂O₃, and r-Cu/Ga₂O₃-SAM at −1.05 V versus RHE. (d) ¹H NMR measurements using ¹⁴NO₃⁻/¹⁵NO₃⁻ and without NO₃⁻ after NO₃RR reactions.
(e) NH₃ yields and FEs of r-Cu/Ga₂O₃-SAM at −1.05 V calculated from NMR and UV–vis, respectively. (f) Long-term durability test of r-Cu/Ga₂O₃-SAM nanofibers at −1.05 V. (g) Comparison of NO₃RR performances with recently reported catalysts in the literature. Operando FT-IR spectroscopy measurements of r-Cu/Ga₂O₃-SAM: (h) at −1.05 V versus RHE over different reaction times, and (i) at different applied voltages.
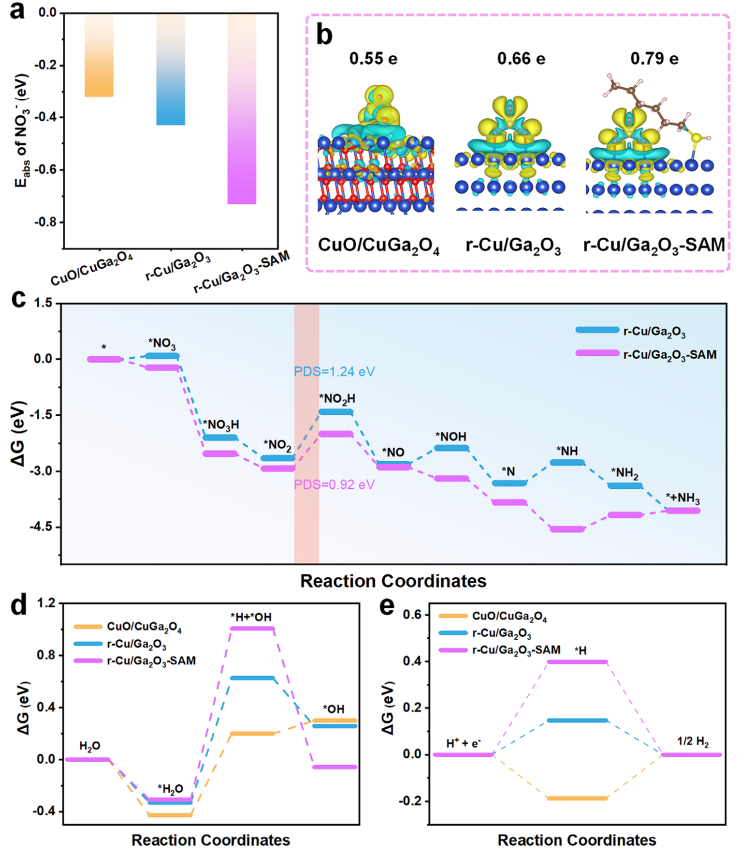
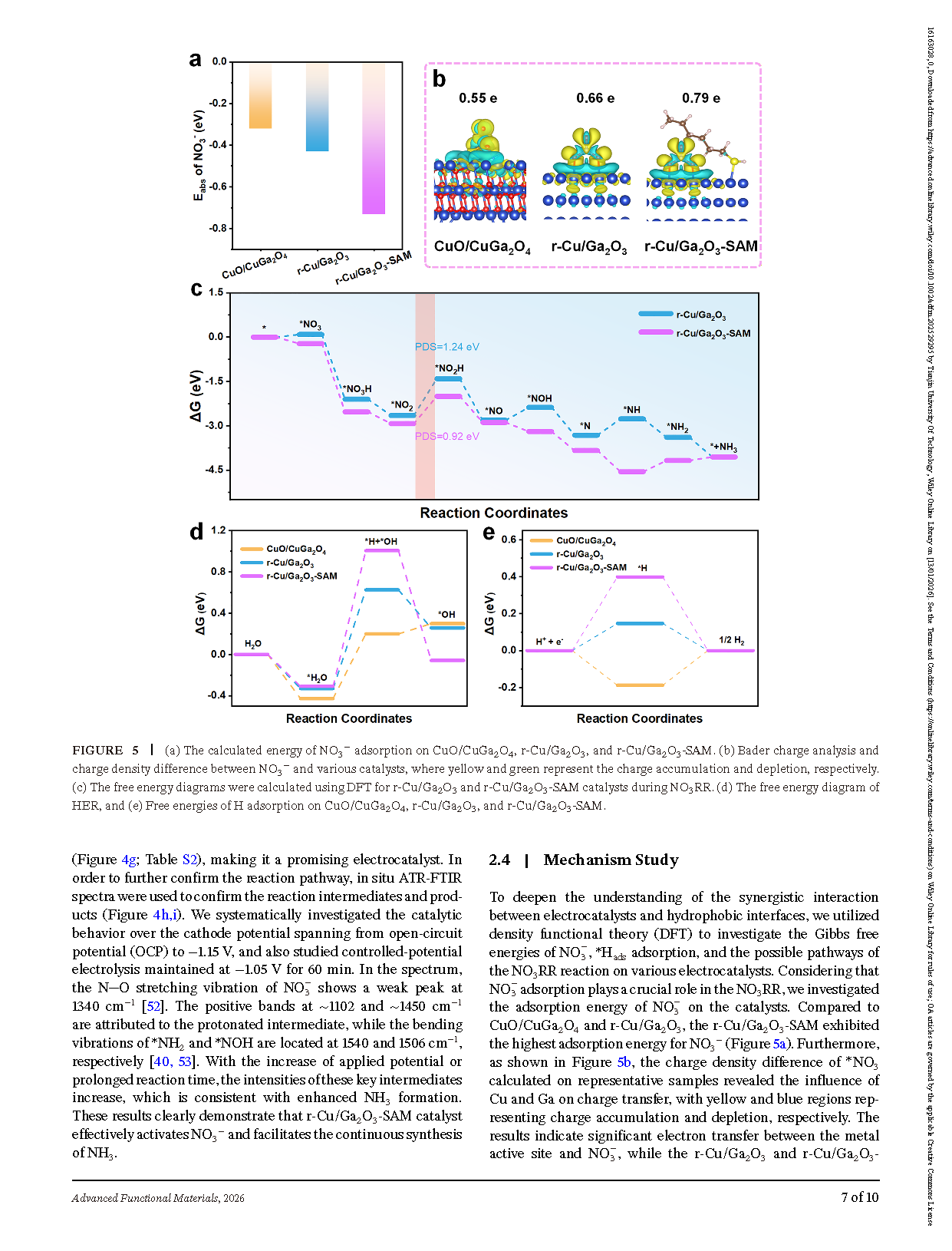
Figure 5. Computational insights into NO₃⁻ adsorption and electrocatalytic activity. (a)Calculated adsorption energies of NO₃⁻ on CuO/CuGa₂O₄, r-Cu/Ga₂O₃, and r-Cu/Ga₂O₃-SAM. (b) Bader charge analysis and charge density differences between NO₃⁻ and various catalysts, where yellow and green indicate charge accumulation and depletion, respectively. (c) Free energy diagrams for NO₃RR on r-Cu/Ga₂O₃ and r-Cu/Ga₂O₃-SAM, calculated using DFT. (d) Free energy diagram for the hydrogen evolution reaction (HER). (e) Free energies of H adsorption on CuO/CuGa₂O₄, r-Cu/Ga₂O₃, and r-Cu/Ga₂O₃-SAM.
DOI:
doi.org/10.1002/adfm.202529295