【Member Papers】Janus-faced role of oxygen vacancies in α-Ga₂O₃: A catalyst design optimization for photocatalytic water splitting
日期:2026-01-26阅读:55
Researchers from the Hong Kong University of Science and Technology (Guangzhou) have published a dissertation titled " Janus-faced role of oxygen vacancies in α-Ga2O3: A catalyst design optimization for photocatalytic water splitting " in Materials Today Chemistry.
Project Support
The work is supported by C. K. Tan start-up fund from Hong Kong University of Science and Technology (Guangzhou), China; Guangzhou Municipal Science and Technology Project (No. 2023A03J0003, No. 2023A03J0013, No. 2023A04J0310 and No. 2023A03J0152); Department of Education of Guangdong Province (No. 2024ZDZX1005); Materials Characterization and Preparation Facility (MCPF) and Green e Materials Laboratory at The Hong Kong University of Science and Technology (Guangzhou). The authors acknowledge financial support from the Shenzhen Science and Technology Innovation Commission (No.20231115111658002). The authors acknowledge financial support from the Shenzhen Science and Technology Innovation Commission (No.20231115111658002).
Background
Solar-driven photocatalytic water splitting is regarded as a promising approach to address the energy crisis and environmental issues, and the development of efficient photocatalysts has become a research hotspot in recent years. Gallium oxide (Ga₂O₃), as a wide-bandgap semiconductor (approximately 4.9 eV), exhibits high mobility of photogenerated charge carriers and excellent activity for water splitting and CO₂ reduction. Although α-Ga₂O₃ is metastable, it is easily synthesized and naturally exists in ambient conditions, and its photocatalytic performance can even surpass that of the more stable β-Ga₂O₃. To enhance catalytic performance, strategies such as cocatalyst deposition, doping, morphology engineering, and heterojunction construction have been employed, among which defect engineering—particularly oxygen vacancies—has proven effective in tuning band structure, active sites, light absorption, and charge transfer. Oxygen vacancies can enhance light absorption and catalytic activity, but excessive or improperly positioned vacancies may inhibit the oxygen evolution reaction (OER) and affect the stability of intermediates, acting as a double-edged sword on catalytic performance. Due to challenges in precisely controlling and characterizing oxygen vacancies experimentally, first-principles calculations based on density functional theory (DFT) have become a vital tool for predicting material properties and exploring microscopic reaction mechanisms. Existing studies on Ga₂O₃ oxygen vacancies primarily focus on the β phase, whereas systematic investigations of oxygen vacancies on the photocatalytic overall water-splitting performance of α-Ga₂O₃—especially on low-index surfaces—remain limited. Therefore, this work employs DFT to systematically investigate the mechanisms of photocatalytic water splitting on α-Ga₂O₃ surfaces with and without oxygen vacancies, analyzing their effects on adsorption behavior, electron transfer, and reaction free energies.
Abstract
α-Ga2O3 has garnered increasing attention due to its unique properties in the field of photocatalysis. However, the mechanistic aspects of photocatalytic water-splitting on α-Ga2O3 surfaces containing oxygen vacancies are still not well understood, hindering the development of high-performance catalysts. Here, we present a comprehensive and systematic investigation into the effects of oxygen vacancies on the photocatalytic water splitting in α-Ga2O3 from structural and electronic perspectives, based on functional theory calculations. In the presence of oxygen vacancies, vacancies at particular sites can lead to structural distortions that significantly improve hydrogen evolution reaction activity. In contrast, oxygen evolution reaction activity is inhibited on both the (001) and (012) surfaces with oxygen vacancies, attributed to the enhanced adsorption strength of intermediates resulting from the electrons associated with the vacancies. This study elucidates the Janus-faced role of oxygen vacancies in α-Ga2O3 during photocatalytic water splitting, offering theoretical guidance for developing novel Ga2O3 catalysts with superior catalytic activity.
Highlights
The pristine α-Ga2O3(012) surface exhibits excellent photocatalytic water-splitting activity.
The Janus-faced role of oxygen vacancies in α-Ga2O3on the photocatalytic water splitting reaction has been revealed.
OV3_1 on (012) surface shows ΔGHof 0.02 eV, near ideal for H2evolution, due to oxygen vacancy-induced distortion.
lOxygen vacancies inhibit O2evolution by enhancing intermediate adsorption via localized electrons.
Conclusion
The photocatalytic overall water-splitting reaction on the (001) and (012) surfaces of α-Ga₂O₃ containing oxygen vacancies at different positions was systematically investigated through theoretical calculations, with the aim of elucidating the intrinsic surface properties and the role of oxygen vacancies in the overall reaction. The double-edged nature of oxygen vacancies in α-Ga₂O₃ during water splitting is revealed. For the hydrogen evolution reaction (HER), the introduction of oxygen vacancies induces structural distortions in the hydrogen adsorption configurations, thereby modulating the reaction activity. A higher degree of structural deformation tends to lower the reaction energy barrier. Notably, the OV3_1 configurations on the (001) and (012) surfaces exhibit remarkable ΔG_H values of 0.06 and 0.02 eV, respectively, which are close to those of optimal HER catalysts. In addition, the HER performance of the defective (001) surface is strongly dependent on the specific vacancy position. In contrast, for the oxygen evolution reaction (OER), only the pristine (012) surface unexpectedly shows a relatively low overpotential of 0.54 V, whereas all oxygen-deficient structures exhibit increased OER overpotentials. Charge density difference analysis and Bader charge calculations reveal that electrons originating from oxygen vacancies significantly enhance the adsorption strength of OER intermediates, leading to inferior OER catalytic activity. Overall, these results indicate that the (012) surface of α-Ga₂O₃ possesses excellent intrinsic catalytic activity for photocatalytic overall water splitting, and they provide theoretical insights and guidance for tuning HER and OER performance via the introduction of oxygen vacancies. Rational design of oxygen vacancy positions to selectively modulate HER and OER activity represents an effective yet challenging strategy.
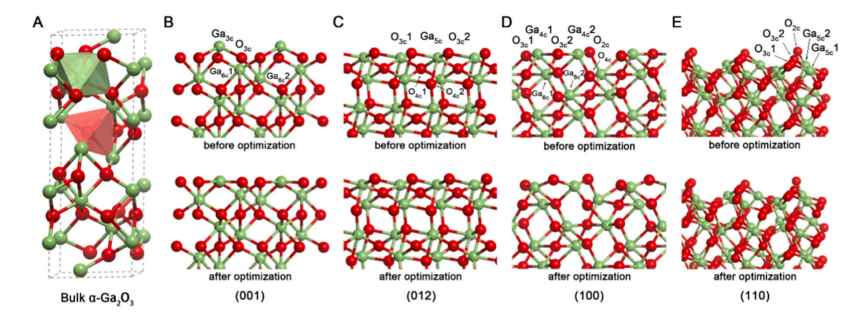
Fig. 1. (A) Optimized bulk structure of α-Ga₂O₃. (B) (001) surface, (C) (012) surface, (D) (100) surface, and (E) (110) surface of α-Ga₂O₃ before and after optimization. Gallium and oxygen atoms are represented by green and red spheres, respectively.
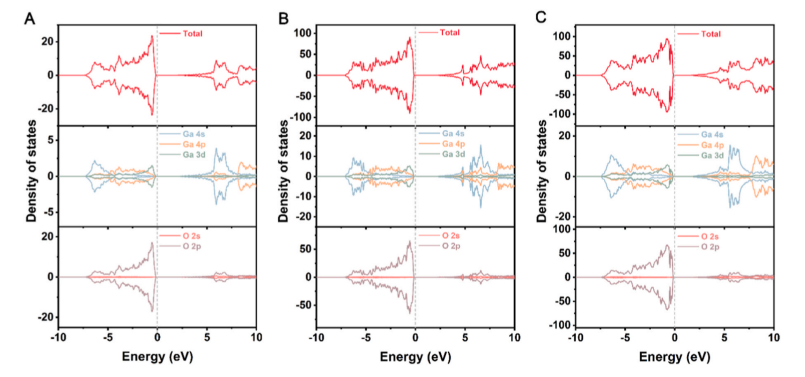
Fig. 2. Density of states (DOS) of (A) bulk α-Ga₂O₃, (B) the (001) surface, and (C) the (012) surface. The light gray dashed line indicates the Fermi level.

Fig. 3. Stable structures and charge density differences for molecular and dissociative water adsorption on α-Ga₂O₃ surfaces: (A, B) (001) surface; (C–E) (012) surface after relaxation. Side views of charge density differences for molecular and dissociative water on: (F, G) (001) surface; (H–J) (012) surface. Yellow regions indicate electron accumulation, while cyan regions indicate electron depletion. The isosurface level is set at 0.004 e⁻/bohr³. One-dimensional planar-averaged charge density differences along the c-axis for: (K, L) (001) surface; (M–Q) (012) surface.

Fig. 4. Schematic diagrams of α-Ga₂O₃ with oxygen vacancies: (A) (001) surface and (B) (012) surface.
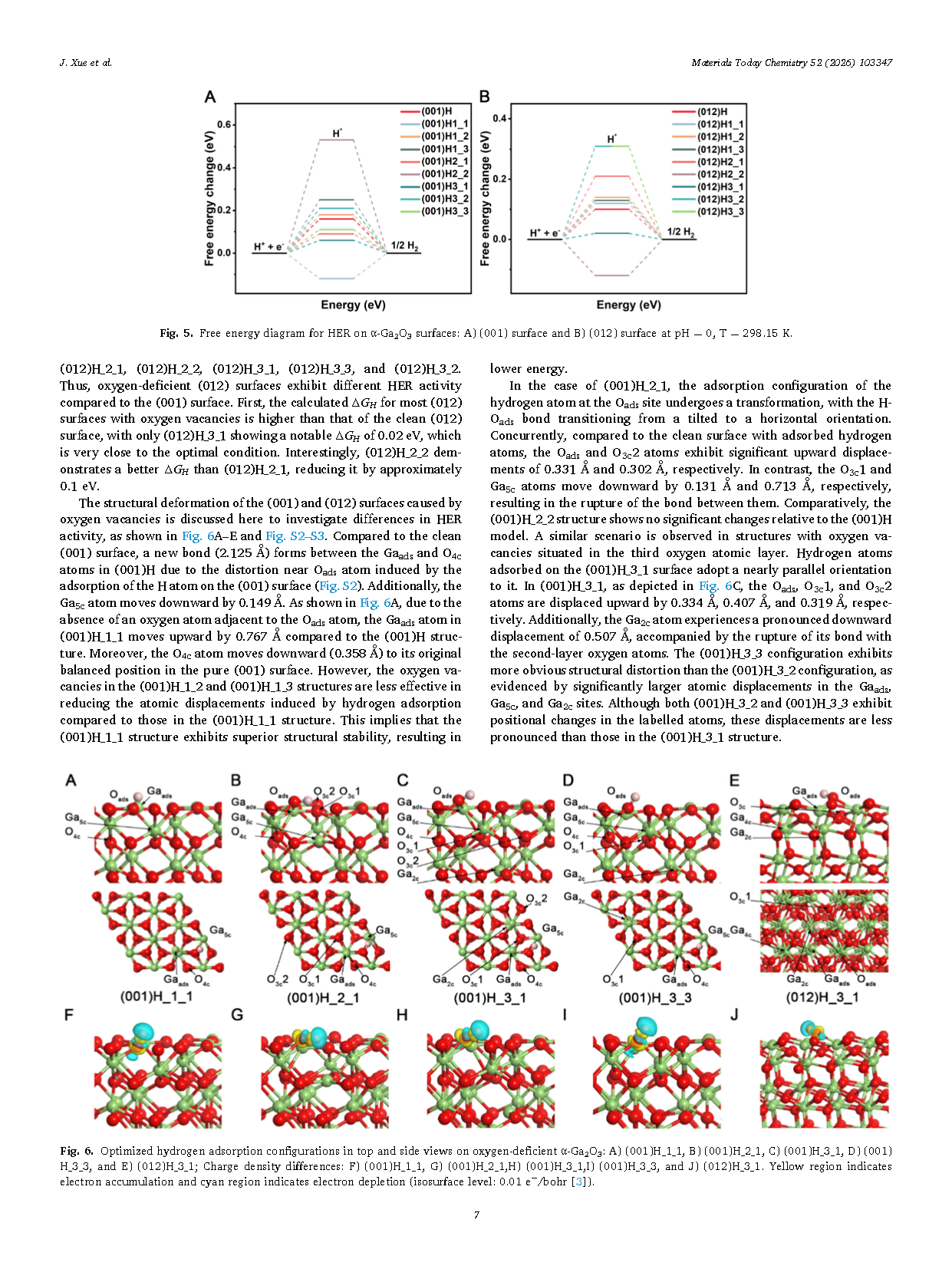
Fig. 5. Free energy diagrams for the hydrogen evolution reaction (HER) on α-Ga₂O₃ surfaces: (A) (001) surface and (B) (012) surface at pH = 0 and T = 298.15 K.

Fig. 6. Optimized hydrogen adsorption configurations on oxygen-deficient α-Ga₂O₃ surfaces: top and side views of (A) (001)H₁₁, (B) (001)H₂₁, (C) (001)H₃₁, (D) (001)H₃₃, and (E) (012)H₃₁. Charge density differences: (F) (001)H₁₁, (G) (001)H₂₁, (H) (001)H₃₁, (I) (001)H₃₃, and (J) (012)H₃₁. Yellow regions indicate electron accumulation, while cyan regions indicate electron depletion. The isosurface level is set at 0.01 e⁻/bohr³.
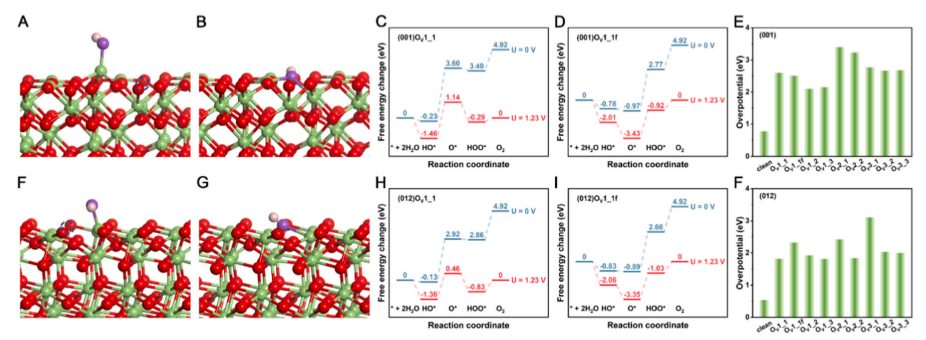
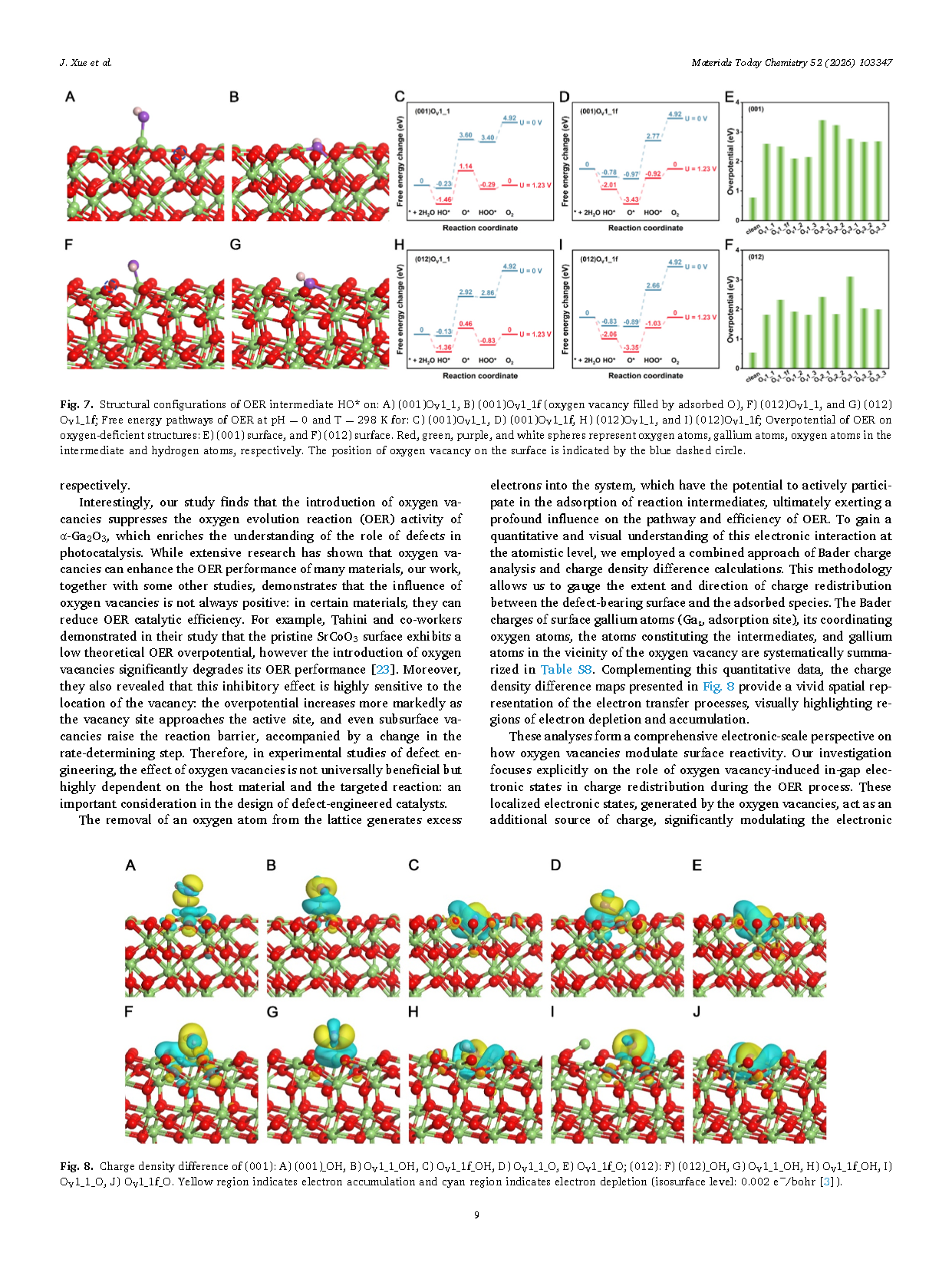
Fig. 7. Structural configurations of OER intermediate HO* on oxygen-deficient α-Ga₂O₃ surfaces: (A) (001)OV₁₁, (B) (001)OV₁₁f (oxygen vacancy filled by adsorbed O), (F) (012)OV₁₁, and (G) (012)OV₁₁f. Free energy pathways of OER at pH = 0 and T = 298 K for: (C) (001)OV₁₁, (D) (001)OV₁₁f, (H) (012)OV₁₁, and (I) (012)OV₁₁f. Overpotentials of OER on oxygen-deficient structures: (E) (001) surface and (F) (012) surface. Red, green, purple, and white spheres represent oxygen atoms, gallium atoms, oxygen atoms in the intermediate, and hydrogen atoms, respectively. The position of the oxygen vacancy on the surface is indicated by the blue dashed circle.
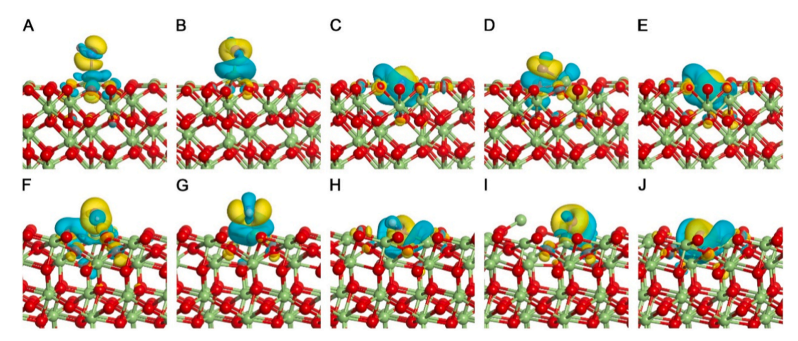
Fig. 8. Charge density differences of α-Ga₂O₃ surfaces: (001) surface—(A) (001)_OH, (B) OV₁₁_OH, (C) OV₁₁f_OH, (D) OV₁₁_O, and (E) OV₁₁f_O; (012) surface—(F) (012)_OH, (G) OV₁₁_OH, (H) OV₁₁f_OH, (I) OV₁₁_O, and (J) OV₁₁f_O. Yellow regions indicate electron accumulation, and cyan regions indicate electron depletion. Isosurface level: 0.002 e⁻/bohr³.
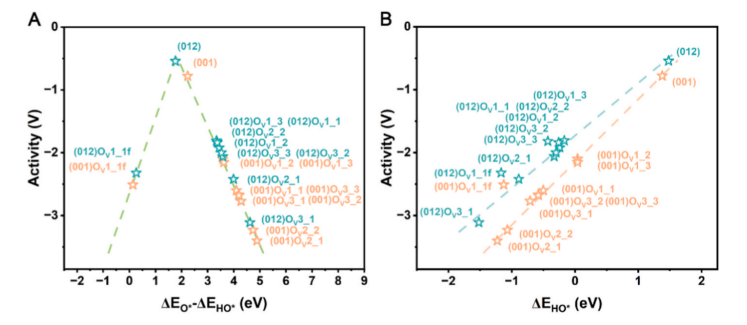
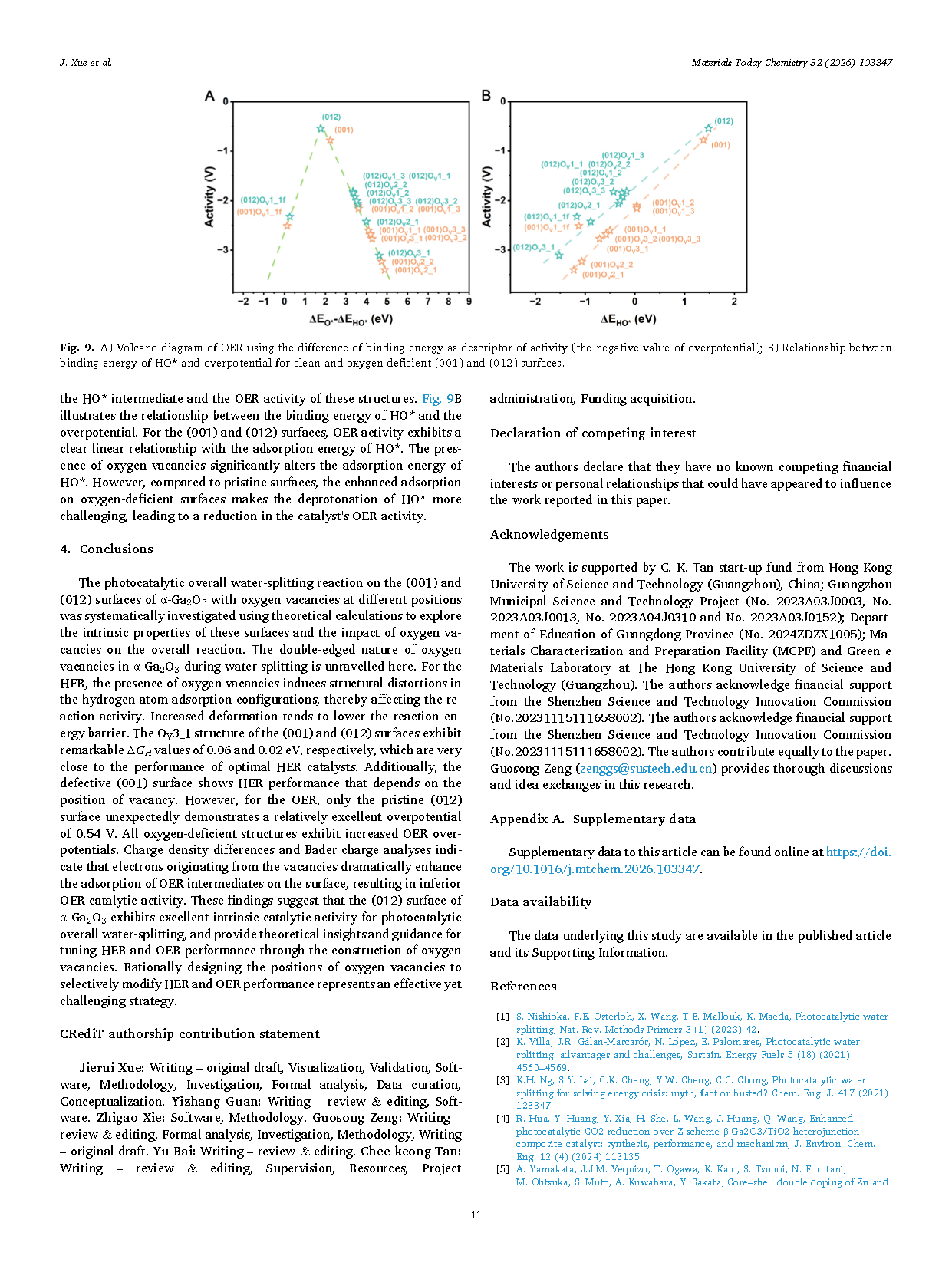
Fig. 9. (A) Volcano plot of the oxygen evolution reaction (OER), using the difference in binding energy as a descriptor of catalytic activity (negative values correspond to overpotential). (B) Relationship between the binding energy of HO* and the OER overpotential for pristine and oxygen-deficient (001) and (012) surfaces.
DOI:
doi.org/10.1016/j.mtchem.2026.103347